In this application note, the Waters Columns Calculator will be used to scale the USP monograph assay method for quetiapine fumarate, an anti-psychotic drug, to smaller particle sized columns.
Users of compendial chromatographic procedures increasingly look to achieve cost savings through analytically equivalent procedures that decrease analysis time and solvent consumption. The United States Pharmacopeia (USP) compendial methods are often written for high performance liquid chromatography (HPLC) columns which use >3 µm particles resulting in long runtimes at higher flow rates. Although USP methods prescribe specific conditions, isocratic methods are capable of being scaled to columns which have a smaller particle size. This results in faster run times and lower solvent consumption. The USP has outlined in the General Chapter <621>1 acceptable column and method adjustments to allow scaling of isocratic methods to provide the same if not improved performance of a method. These allowable adjustments include scaling particle size and column dimensions to maintain the L/dp ratio, where L is the length of the column and dp is the diameter of the particle size of the column packing material, as well as adjusting the flow rate and injection volumes accordingly. For these scaled methods it is important to consider the impact of dwell volume and extra-column dispersion on the chromatographic separation, and whether or not a lower dispersion, higher pressure system such as a UHPLC or a UPLC system is preferred.
In this study, the Waters Columns Calculator will be used to scale the USP monograph assay method for quetiapine fumarate,2 an anti-psychotic drug, to smaller particle sized columns. These resulting methods will then be run on a variety of LC systems. The LC system platforms that will be included are an Alliance HPLC System, an ACQUITY Arc UHPLC System, and an ACQUITY UPLC H-Class PLUS System. We will demonstrate improvements to method performance, decreased run times and improved throughput while achieving the system suitability requirements as stated in the USP monograph for the quetiapine fumarate assay.
Two reference standards were obtained from the USP: Quetiapine System Suitability (Catalog#: 1592715), and the Quetiapine Fumarate Standard (Catalog#: 1592704). The unknown sample was obtained from Alibaba.com. All samples were diluted in mobile phase to the following concentrations: 1.0 mg/mL for the system suitability solution and 0.08 mg/mL for the standard solution and sample solution.
|
Column temp.: |
25 °C |
|
Sample temp.: |
4 °C |
|
Mobile phase: |
Methanol, acetonitrile, and buffer (54:7:39) Premixed and filtered using a 0.45 μm filter |
|
Buffer: |
2.6 g/L of dibasic ammonium phosphate adjusted to pH 6.5 with phosphoric acid |
|
Gradient: |
Isocratic (premixed mobile phase) |
|
PDA wavelength: |
230 nm at 4.8 nm resolution |
|
LC system 1: |
Alliance e2695 Separations Module with 100 μL syringe, 2998 PDA Detector and CH-30 equipped with a passive preheater |
|
Column: |
XBridge BEH C8 Column, 5 μm, 4.6 mm x 250 mm (p/n: 186003018) |
|
Injection volume: |
50 μL |
|
Flow rate: |
1.3 mL/min |
|
Run time: |
15 minutes |
|
Max. system pressure: |
3850 psi |
|
LC system 2: |
ACQUITY Arc System with active solvent preheating (CH-30A) and 2998 PDA Detector (Path 2) |
|
Column: |
XBridge BEH C8 Column, 3.5 μm, 3.0 mm x 150 mm (p/n: 186003052) |
|
Injection volume: |
12.8 μL |
|
Flow rate: |
0.8 mL/min |
|
Run time: |
7 minutes |
|
Max. system pressure: |
5200 psi |
|
LC system 3: |
ACQUITY UPLC H-Class PLUS System with active solvent preheating (CH-30A), 50 μL extension loop, and ACQUITY UPLC PDA Detector |
|
Column: |
ACQUITY UPLC BEH C8 Column, 1.7 μm, 2.1 mm x 75 mm (p/n: 186005606) |
|
Injection volume: |
3.1 μL |
|
Flow rate: |
0.5 mL/min |
|
Run time: |
3 minutes |
|
Max. system pressure: |
14,000 psi |
Empower 3 Chromatography Data Software, FR 3
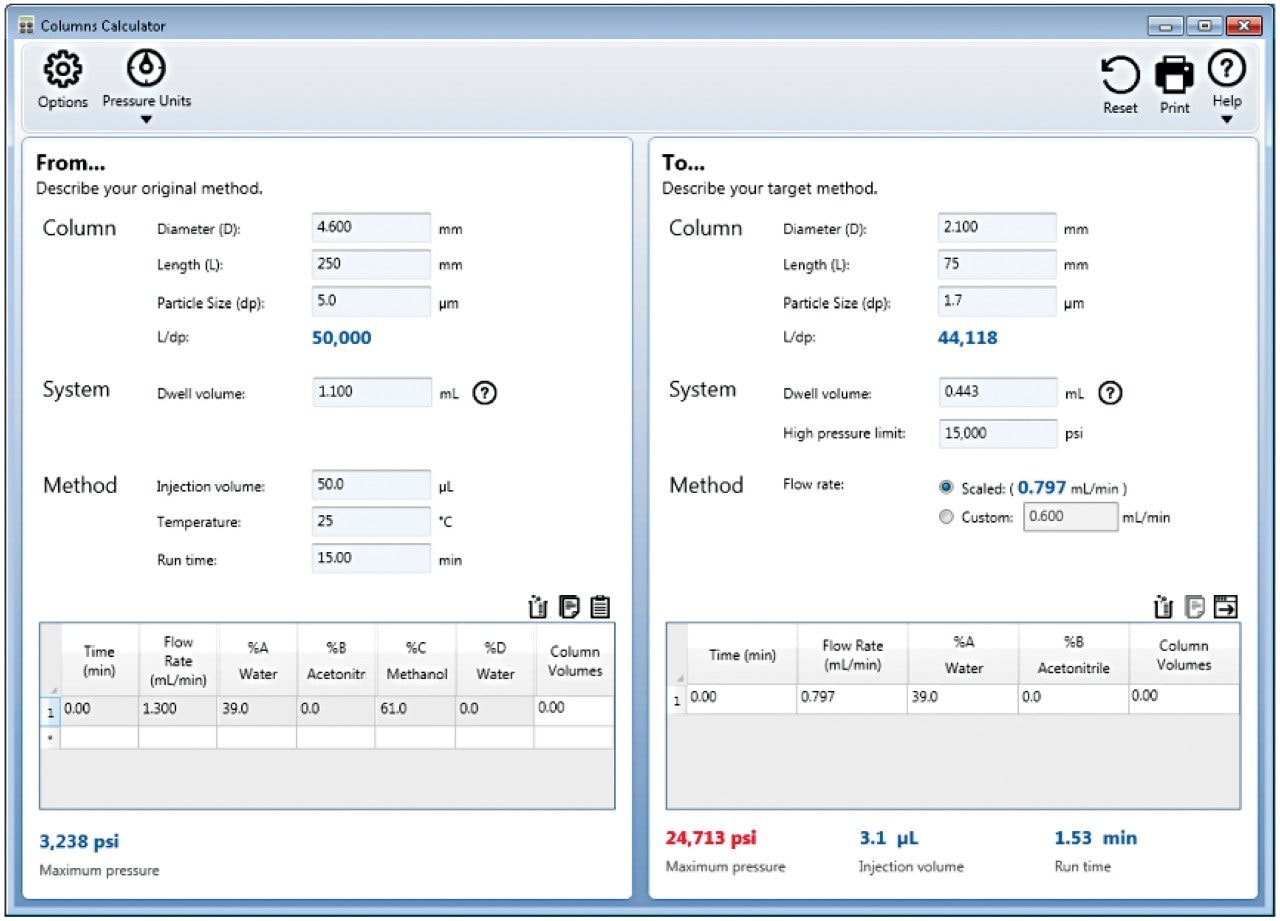
The USP assay for quetiapine fumarate was initially run on the Alliance HPLC System following the column and method details described in the monograph. The column dimensions and method conditions were then scaled according to the USP guidelines1 using the Waters Columns Calculator (Figure 2), and subsequently run on the ACQUITY Arc UHPLC System and the ACQUITY UPLC H-Class PLUS System. Both the column dimensions and the features of an LC system should be used to determine the optimal combination for running a method. Two key attributes of an LC system that should be considered are the dwell volume and the extra-column dispersion. The dwell volume of a system consists of the amount of volume from where the solvents are mixed until the mixed solvents contact the column inlet. While this may have a significant impact for gradient methods, dwell volume has very little effect on isocratic methods.4 Therefore, the extra-column dispersion is the main factor in determining the optimum LC system for a column with an isocratic method. It is important to reduce the extra-column dispersion when using smaller columns since it will affect the resolution of the compounds, the broadening of peaks, and the efficiency of the column.5 Therefore, to achieve optimal performance, it is important to keep the extra-column dispersion as minimal as possible in comparison to the column volume. For a more in depth breakdown of dwell volume and extra-column dispersion refer to the white paper listed in reference #5.
To maintain the L/dp ratio the column dimensions and particle size were scaled from the original column dimensions to a column with a 3.5 µm particle size and 3.0 mm × 150 mm column dimensions (Figure 2). In order to match the lower column volume with a lower dispersion system, the analysis of the quetiapine fumarate was performed on the ACQUITY Arc UHPLC System. The L/dp ratio decreased by 14% but is within the USP criteria of -25% to +50% of the original method. When the particle size and diameter of a column are changed, the following equation is used to calculate the change in flow rate:
F2 = F1 × [(dc22 × dp1)/( dc12 × dp2)]
where F1 and F2 are the flow rates for the original and scaled method, respectively; dc1 and dc2 are the column diameters for the original and scaled method, respectively, and dp1 and dp2 are the diameters of the particle sizes of the original and scaled methods, respectively.1 The flow rate for this experiment was decreased from 1.3 mL/min in the original method to 0.8 mL/min for the 3.5 µm particle size column method. According to the USP general guidelines, “the injection volume for a method may be adjusted as long the value is within the accepted precision, linearity, and detection limits”.1 The injection volume was decreased from the original method of 50 µL to 12.8 µL for the scaled method conditions and was determined using the Waters Column Calculator (Figure 2).

In order to scale the quetiapine fumarate assay to a 1.7 µm particle column, the Waters Columns Calculator was utilized once again (Figure 3). The analysis of the quetiapine fumarate on the 2.1 mm × 75 mm column was performed on the ACQUITY UPLC H-Class PLUS System in order to decrease the extra-column dispersion that would be present on the Alliance HPLC System and ACQUITY Arc UHPLC System. The scaled column dimensions resulted in an L/dp ratio decrease of 12%, however, are within the USP criteria of -25% to +50% of the original method. The flow rate was decreased from 1.3 mL/min to 0.8 mL/min and the injection volume was decreased from 50 µL to 3.1 µL when scaling from the original method to the 1.7 µm particle size column. The flow rate was not run at 0.8 mL/min as stated in the Columns Calculator (Figure 3) because the ACQUITY UPLC H-Class PLUS System was over the 15,000 psi pressure limit at that flow rate. The final flow rate of 0.5 mL/min was used since the maximum system pressure achieved was 14,000 psi. This flow rate adjustment is acceptable under the USP conditions stated in the General Chapter <621> that once the flow rate is adjusted accordingly to the column, the flow rate can then be additionally adjusted within ± 50%.1
All samples were prepared as described in the experimental conditions according to the USP assay with six replicate injections for each solution. In order to determine the successful performance of the scaled methods run on the appropriate chromatographic system, the system suitability requirements stated in the quetiapine fumarate USP monograph were evaluated. Additionally, an unknown sample was analyzed on each of the three systems to determine the reproducibility of the scaled methods.
System suitability tests are an important component of the USP monograph given that they determine whether or “not a chromatographic system is adequate for an intended analysis”.3 For the system suitability, the USP monograph requires that the resolution between the quetiapine desethoxy and the quetiapine peaks (Figure 4) must be not less than (NLT) 1.5, with peak identification based on relative retention times contained in the USP monograph. The standard solution requires the tailing factor be not more than (NMT) 2.0 and the relative standard deviation (RSD) be NMT 2.0%. The first peak of the chromatogram for all samples pertains to the fumaric acid and was not considered when evaluating the system suitability requirements for retention time, area, and tailing since it is not the main active pharmaceutical ingredient (API).
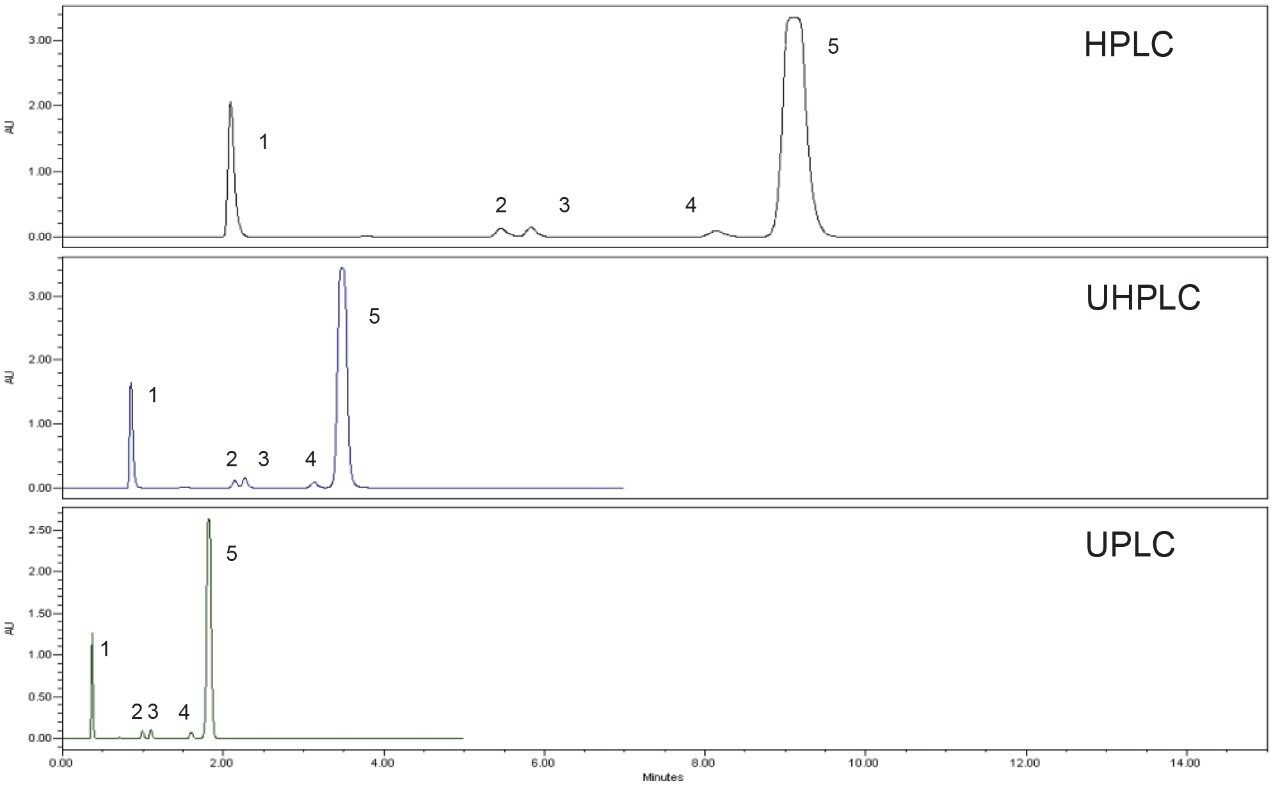
The USP assay method for quetiapine fumarate was evaluated using the original and scaled methods run on the prescribed LC systems. Example chromatograms obtained using the three methods are shown in Figure 4 (system suitability) and Figure 5 (sample solution). All methods met the system suitability requirements for both the system suitability solution and the standard solution with the results located in Table 1.
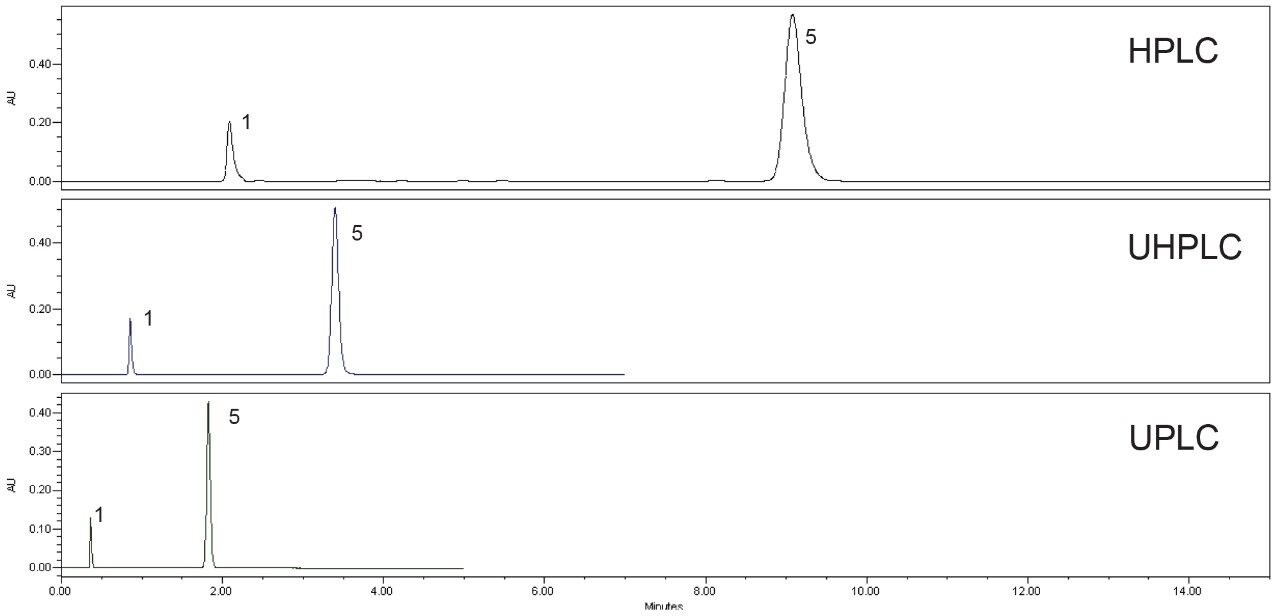

In addition to providing similar or improved chromatographic performance, scaling the original method to a smaller particle column also significantly decreased the run time and solvent consumption. Scaling the original method to a 3.5 µm column decreased the run time from 15 minutes to 7 minutes, and decreased the solvent usage by 66%. Further scaling to a 1.7 µm column decreased the run time from 15 minutes to approximately 2 minutes and reduced the solvent usage by almost 90%. Scaling USP methods to run on smaller particle columns can drastically increase throughput while decreasing cost per sample.
Also, an unknown sample was analyzed in order to determine the percent of quetiapine fumarate present. The standard solution and the sample solution data were used to calculate the percent as follows:
Result = (ru/rs) × (Cs/Cu) × 100
where ru is the peak response from the sample solution, rs is the peak response from the standard solution, Cs is the concentration of USP quetiapine fumarate standard in the standard solution (mg/mL), and Cu is the concentration of quetiapine fumarate in the sample solution (mg/mL).2 The calculated percent of quetiapine fumarate generated using the different methods can be found in Table 2. These values do not fall within the USP acceptance criteria of 98.0% to 102.0%. If this sample were to be used for batch release, a further investigation would be required to determine the source of the over-estimation of the purity. This may include evaluation of peak purity or possibly analysis by an orthogonal detection mechanism such as mass spectrometry. However, since the aim of this application note is to demonstrate the scalability of the isocratic method, further evaluation is outside the scope of this work.
An important part of method scaling is the ability to generate the same results using either the original or the scaled method conditions. Scaling the USP quetiapine assay method across the different LC systems produced consistent results for the unknown sample (Table 2). This demonstrates that scaled methods can be used to generate reliable data.

Utilizing the method scaling guidelines within the USP General Chapter <621>,1 traditional isocratic HPLC methods are capable of being scaled to columns with smaller particle sizes and shorter lengths. Scaling a USP method enables the use of modern chemistries and LC hardware to deliver improved throughput with decreased solvent consumption all while providing accurate and reproducible chromatographic data. The scaled modifications for the USP quetiapine fumarate assay method enabled the original method run time to be reduced by 57% for a 3 µm column on an ACQUITY Arc UHPLC System and 87% reduction for the 1.7 µm column on an ACQUITY UPLC H-Class PLUS System. The scaled methods maintained the same or improved chromatographic performance in terms of resolution, peak tailing, and retention time and peak area RSD.
720006291, June 2018