Rapid Mixed-Mode SPE Method Development Using the Oasis® µElution Sorbent Selection Plate
Rapid Mixed-Mode SPE Method Development Using the Oasis® µElution Sorbent Selection Plate
Ce document est une note d’application et ne contient pas de section détaillée concernant l’expérimentation.
Abstract
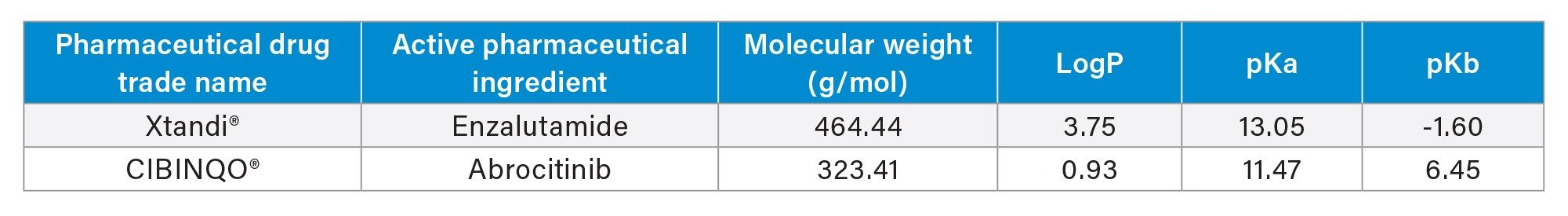
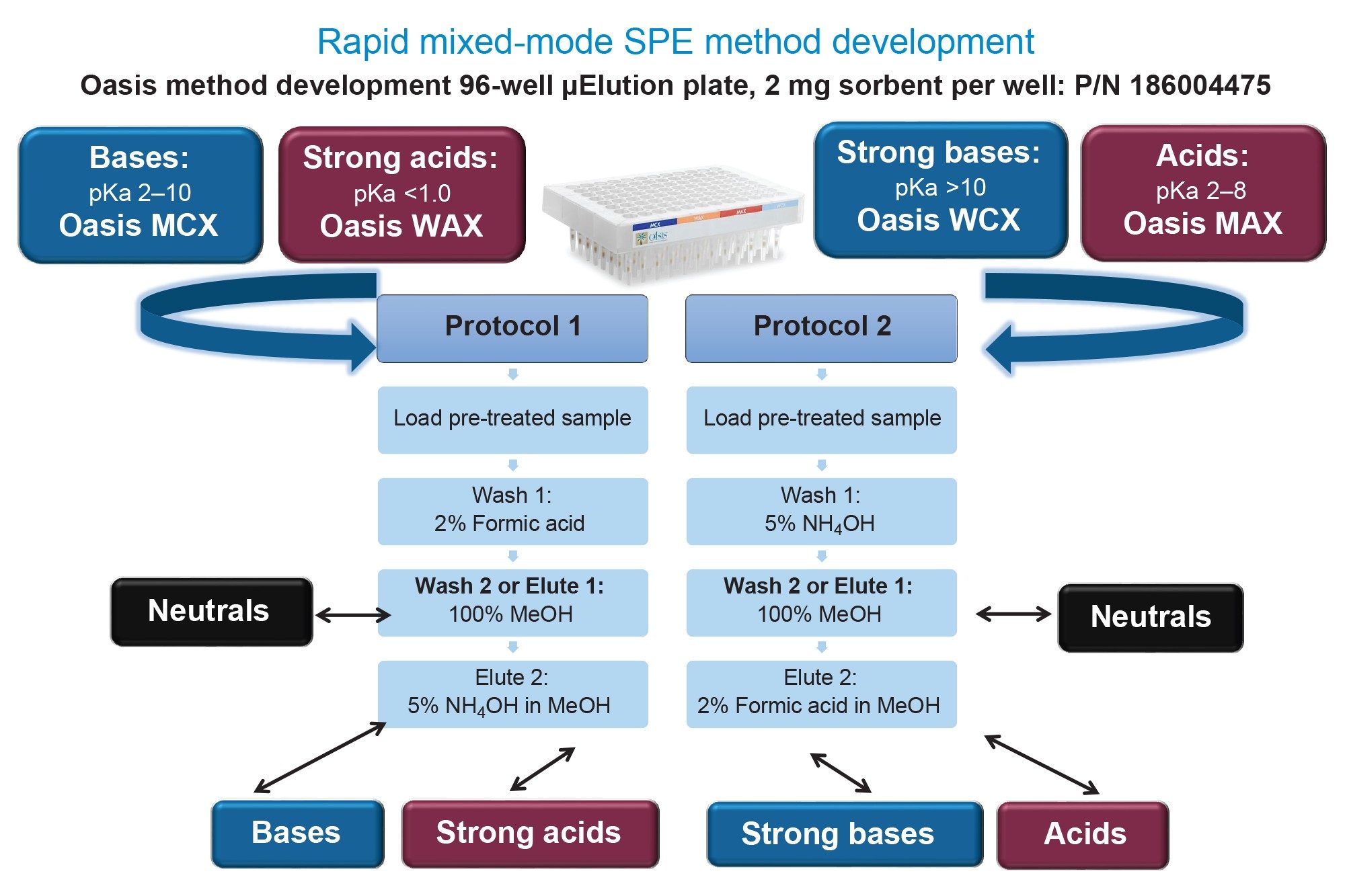
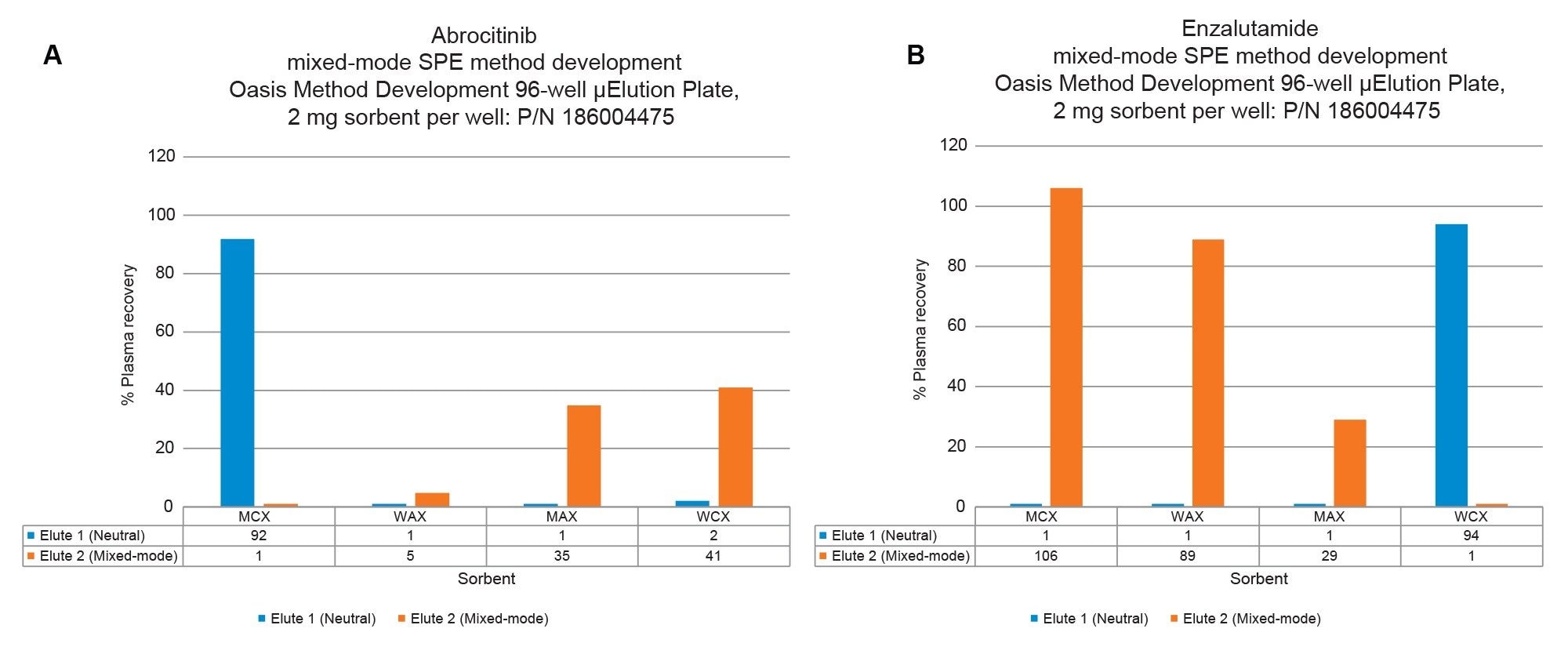
This application demonstrates the fast and simple mixed-mode (MM) solid phase extraction (SPE) method development using the Oasis Method Development μElution Plate, which contains 4 sorbent chemistries (MCX, WAX, WCX, MAX), facilitating rapid screening in one experiment with only 2 protocols for the pharmaceuticals, abrocitinib, and enzalutamide.
Experimental
Results and Discussion
The Oasis Method Development µElution Plate and starting protocols provide quick and easy mixed-mode SPE method development in one experiment, facilitating high analyte recovery, and selectivity (low matrix effects) from plasma.
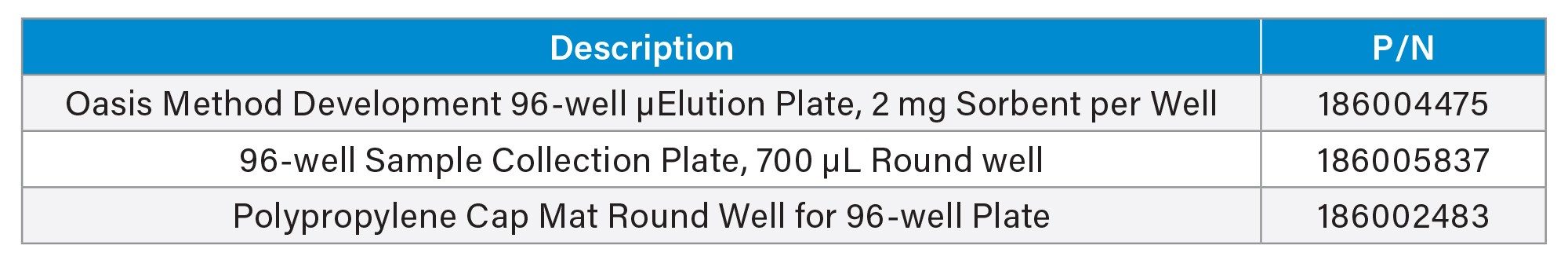
Ordering Information
720008425, July 2024