In early 2015, the U.S. FDA announced that it was investigating reports that low levels of a common fungicide, carbendazim, were being detected in imported orange juice. Naringin, a flavanone commonly found in very low levels in orange juice and high levels in grapefruit juice, is commonly tested to ensure that the orange juice is not being adulterated with grapefruit juice. At the time of writing, the testing limits for these compounds were 10 ppm for carbendazim and 0.1 ppm for naringin.
Time-consuming HPLC/UV methods are deployed in testing labs as a first pass test for these compounds. These methods are neither sensitive nor selective and therefore samples are typically re-directed to centralized MS/MS labs for further testing when any peak elutes near the expected retention time.
In this application note, learn how mass detection can be deployed in food QC lab environments to provide the selectivity and sensitivity necessary to detect low levels of compounds in complex matrices, such as orange juice.
The sensitivity challenges of the aforementioned HPLC-UV method can benefit from multi-dimensional UPLC, which is an emerging technology that can provide increased peak capacity and increased sensitivity. One approach to multi-dimensional chromatography is to use trap-and-elute with at-column dilution to focus large amounts of material on a trap and then to elute to a chromatography column. Sample enrichment can be achieved as a result of deploying this approach in the lab.
Regulatory agencies have been testing orange juice for pesticide residues and adulteration for decades. In early 2015, the U.S. FDA announced that it was investigating reports that low levels of a common fungicide, carbendazim, were being detected in imported orange juice. Naringin, a flavanone commonly found in very low levels in orange juice and high levels in grapefruit juice, is commonly tested to ensure that the orange juice is not being adulterated with grapefruit juice. At the time of writing, the testing limits for these compounds were 10 ppm for carbendazim and 0.1 ppm for naringin.1 Time-consuming HPLC/UV methods are deployed in testing labs as a first pass test for these compounds. These methods are neither sensitive nor selective and therefore samples are typically re-directed to centralized MS/MS labs for further testing when any peak elutes near the expected retention time.
To address the challenges for routine testing, a simplified, easy-to-use mass detection method can be used. Waters ACQUITY QDa Mass Detector is a compact single quadrupole mass spectrometer that is as intuitive and as easy to use as an optical detector. It is pre-optimized to work with most samples without the tuning or sample specific optimization necessary for most mass spectrometers. Mass detection can be deployed in QC lab environments to provide the selectivity and sensitivity necessary to detect low levels of compounds in complex matrices, such as orange juice.
The sensitivity challenges of the aforementioned HPLC-UV method can benefit from multi-dimensional UPLC, which is an emerging technology that can provide increased peak capacity and increased sensitivity. One approach to multi-dimensional chromatography is to use trap-and-elute with at-column dilution to focus large amounts of material on a trap and then to elute to a chromatography column. Sample enrichment can be achieved as a result of deploying this approach in the lab.
Standards were prepared from 100 ppm naringin in water and 100 ppm carbendazim in methanol standard stock solutions. A matrix blank was prepared by centrifuging a sample of low pulp orange juice for 20 minutes in a bench top centrifuge and collecting the supernatant. A 10 ppm solvent standard was prepared by mixing 100 µL of each standard stock solution with 800 µL of water and vortexing to mix. Lower concentration solvent standards were prepared by serial dilution with water. A combined 10 ppm matrix standard was prepared by mixing 100 µL of each standard stock solution with 800 µL of matrix blank and vortexing to mix. Lower concentration matrix standards were prepared by serial dilution with matrix blank.
For analysis, each solvent standard and matrix standard was diluted and filtered before injection. A 100 µL aliquot of each standard was subsequently mixed with 300 µL of mobile phase B (10 mM ammonium acetate in methanol) and 600 µL of mobile phase A (10 mM ammonium acetate in water), vortexed and filtered through a 0.2-µm GHP filter (Waters Acrodisc Minispike Syringe Filter, GHP, 13-mm 0.2-µm, WAT097962). A 2-µL aliquot was injected using a standard 15-µL needle and 100-µL syringe. For injections over 10 µL, a 50-µL extension loop was with the previously described configuration.
|
UPLC System: |
ACQUITY UPLC H-Class with CM-A |
|
Detector: |
PDA and ACQUITY QDa (in series) |
|
Column: |
ACQUITY UPLC BEH C18, 1.7 μm , 2.1 x 150 mm (P/N: 186002353) |
|
Mobile phase A: |
10 mM Ammonium acetate in water |
|
Mobile phase B: |
10 mM Ammonium acetate in methanol |
|
Seal wash: |
90/10 Water/methanol |
|
Purge/needle wash: |
50/50 Water/methanol |
|
Flow rate: |
0.4 mL/min |
|
Isocratic flow: |
61/39 A:B |
|
Run time: |
3 min |
|
Injection volume: |
2 μL |
|
Sample temp.: |
4 °C |
|
Column temp.: |
60 °C |
|
Detector: |
ACQUITY UPLC ePDA |
|
Wavelength: |
254 nm |
|
Sampling rate: |
10 pts/sec |
|
UPLC System: |
ACQUITY UPLC H-Class with 2D Technology, with at-column dilution |
|
Detector: |
PDA and ACQUITY QDa (in series) |
|
Column: |
ACQUITY UPLC BEH C18, 1.7 μm, 2.1 x 150 mm (P/N: 186002353) |
|
Trap column: |
XBridge C18 Direct Connect HP, 10 μm, 2.1 x 30 mm (P/N: 186005232) |
|
QSM (1st dimension pump): |
0.2 mL/min 10 mM Ammonium acetate in water |
|
ISM (dilution pump): |
1.8 mL/min 10 mM Ammonium acetate in water |
|
BSM (elution pump): |
0.4 mL/min, 61/39 Mobile phase A/B as above |
|
All other conditions are per the LC conditions (1D). |
|
MS system: |
ACQUITY QDa |
|
Ionization modes: |
ESI+ (carbendazim) and ESI- (naringin) |
|
Probe temp.: |
300 °C |
|
Capillary voltages: |
1.5kV (ESI+), 0.8kV (ESI-) |
|
Sampling rate: |
5 pts/sec |
|
Acquisition range: |
100 to 600 m/z |
|
Name |
RT (min) |
ESI mode |
SIR (par) |
CV (par) |
SIR (frag) |
CV (frag) |
|
Carbendazim |
2.19 |
ESI+ |
192.04 m/z |
10 V |
159.97 m/z |
40 V |
|
Naringin |
2.38 |
ESI- |
579.07 m/z |
25 V |
270.83 m/z |
60 V |
Multi-dimensional chromatography is possible on the ACQUITY UPLC H-Class System by using a CM-A with the optional 6-port valves. Extra dilution/elution pumps can be configured with the ACQUITY UPLC H-Class System using either Empower 3 or MassLynx Software. Trap-and-elute is a technique that allows for focusing material on a first dimension “trap” column before transferring to the second dimension “chromatography” column for analysis. This can be useful to capture sections of an existing chromatogram or to aid in sample enrichment (or both). At-column dilution (ACD) is a technique that allows for large volume injections of relatively strong sample diluents. By tee-ing in a second low organic mobile phase at a larger flow rate using a dilution pump, the slug of injected material can be easily refocused onto the head of a column (Figure 1a). When used in combination with trap-and-elute, an elution pump can be used to back-flush the focused band of material from the trap for separation on a UPLC column (Figure 1b). An XBridge C18 Direct Connect HP Column was used to trap-and-elute large volumes of naringin and carbendazim before transferring the material to the UPLC column in an attempt to improve the sensitivity of the method.
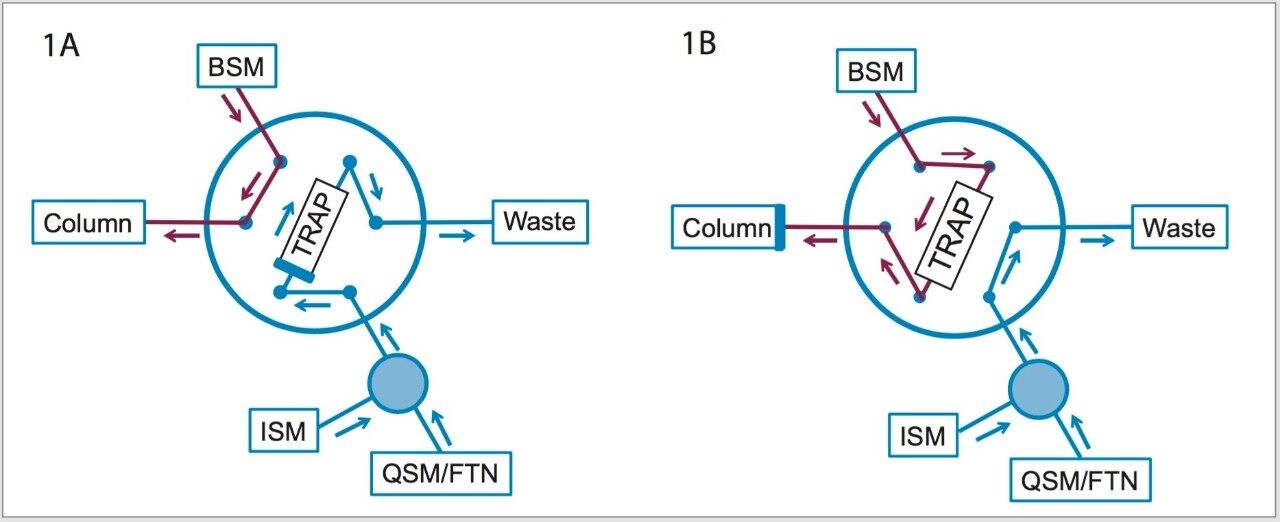
Figure 1A. Picture of tubing layout.
Figure 1B. Picture of tubing layout.
For both methods, simultaneous positive and negative single ion recordings were collected at the m/z values previously described. The cone voltage was optimized for the parent base ion and then pushed to high levels to induce in-source fragmentation of each compound of interest. The response of the parent ion and the fragment are consistent from injection to injection and so were compared to aid in peak identification.
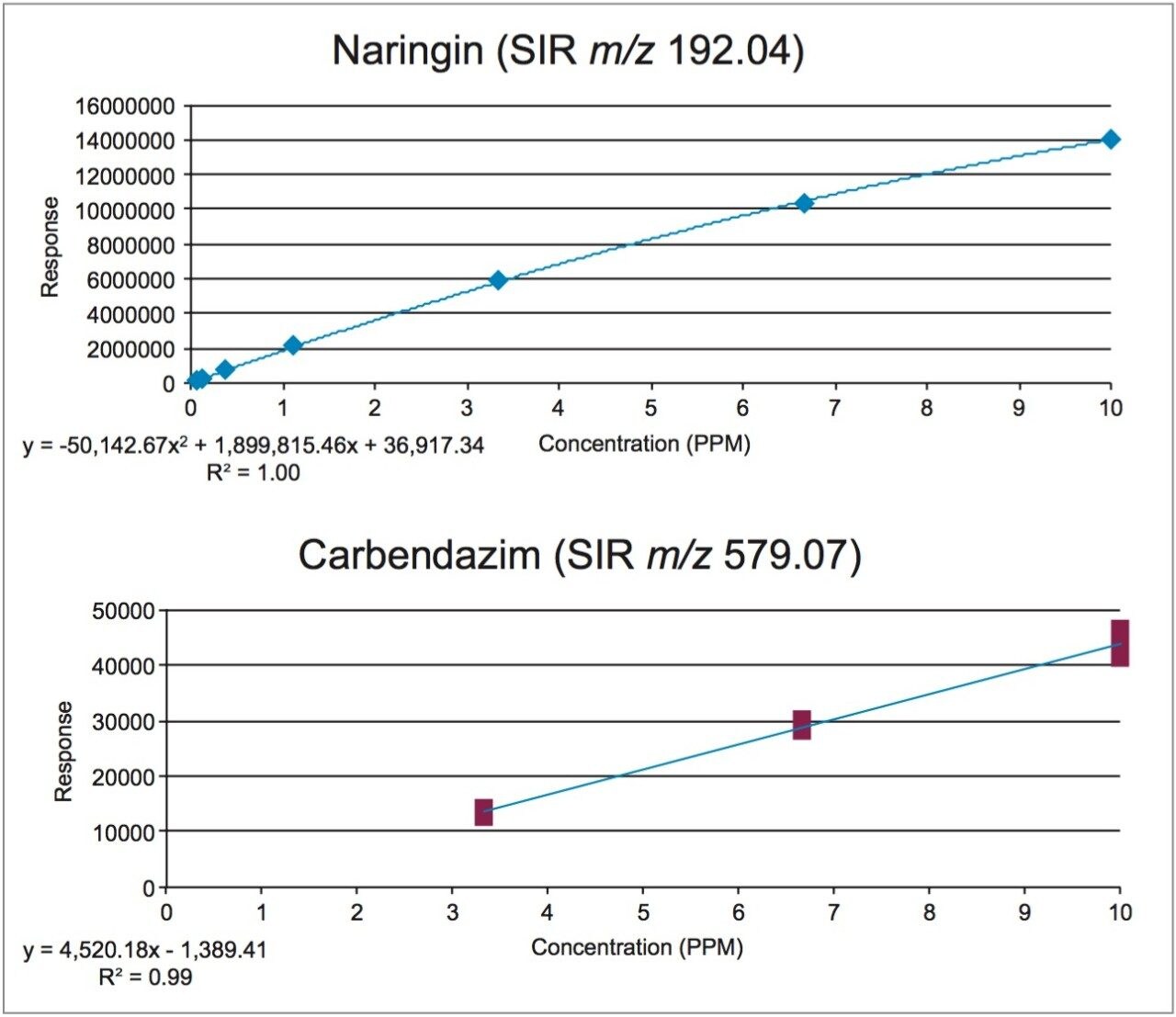
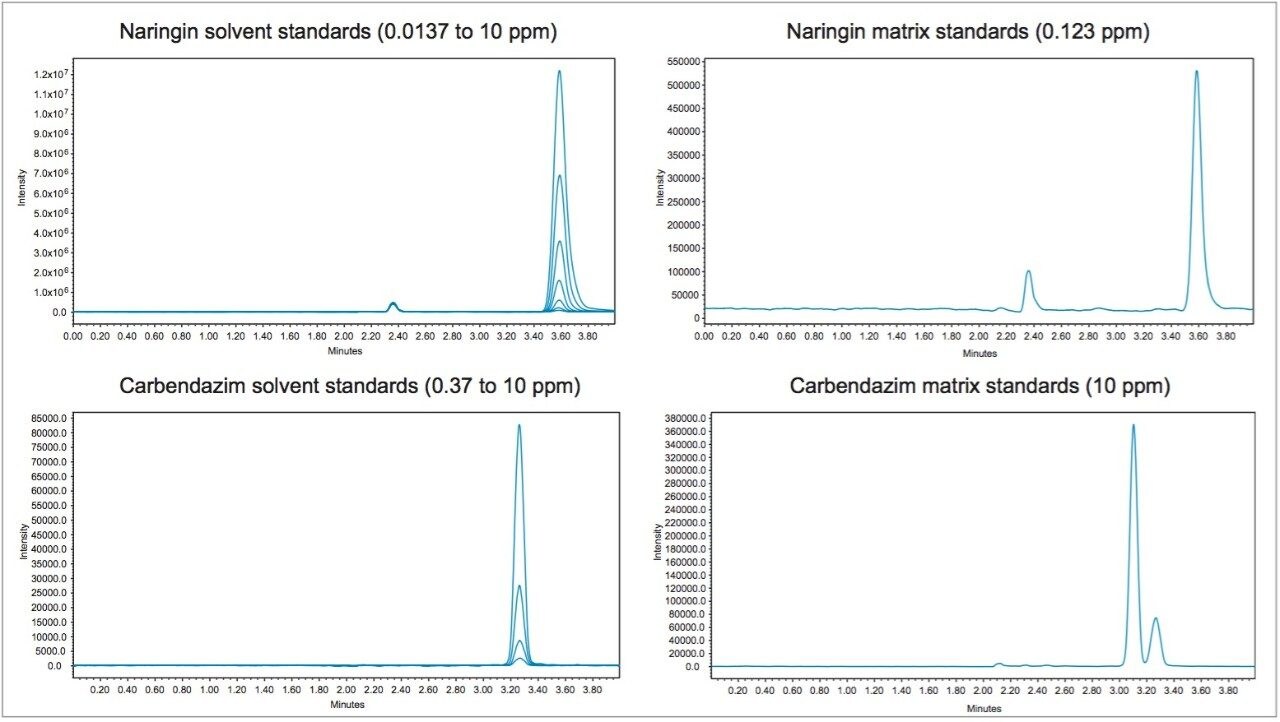
Under isocratic conditions, calibration curves were prepared for both analytes using mass detection. Solvent standards were prepared in the range of 0.0412 ppm to 10 ppm (Table 1). Calibration curves were produced for 3.33 to 10 ppm for carbendazim (linear, R2 = 0.99) and 0.0412 to 10 ppm for naringin (quadratic, R2 = 1.00) (Figure 2). Matrix standards were prepared at 0.123 ppm and 10 ppm levels. Precision was tested for the solvent and matrix standards at the required limits of 0.123 ppm (naringin) and 10 ppm (carbendazim). Precision was found to be 1.1% (solvent) and 3.3% (matrix) for naringin and 4.7% (solvent) and 5.0% (matrix) for carbendazim. These results met the previously described limits.
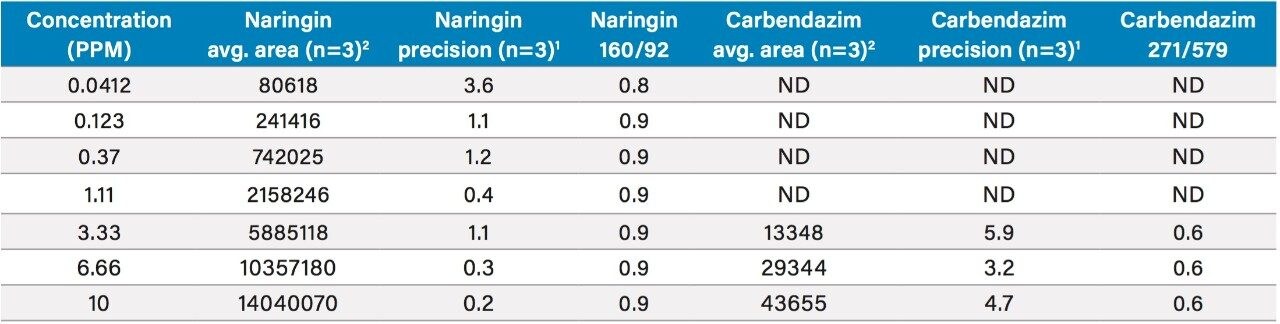
Table 1. Calibration data for solvent standards.
1n=6 at 0.123% and 10.0% levels.
2Naringin area uses SIR m/z 192.04, Carbendazim data uses SIR m/z 579.07.
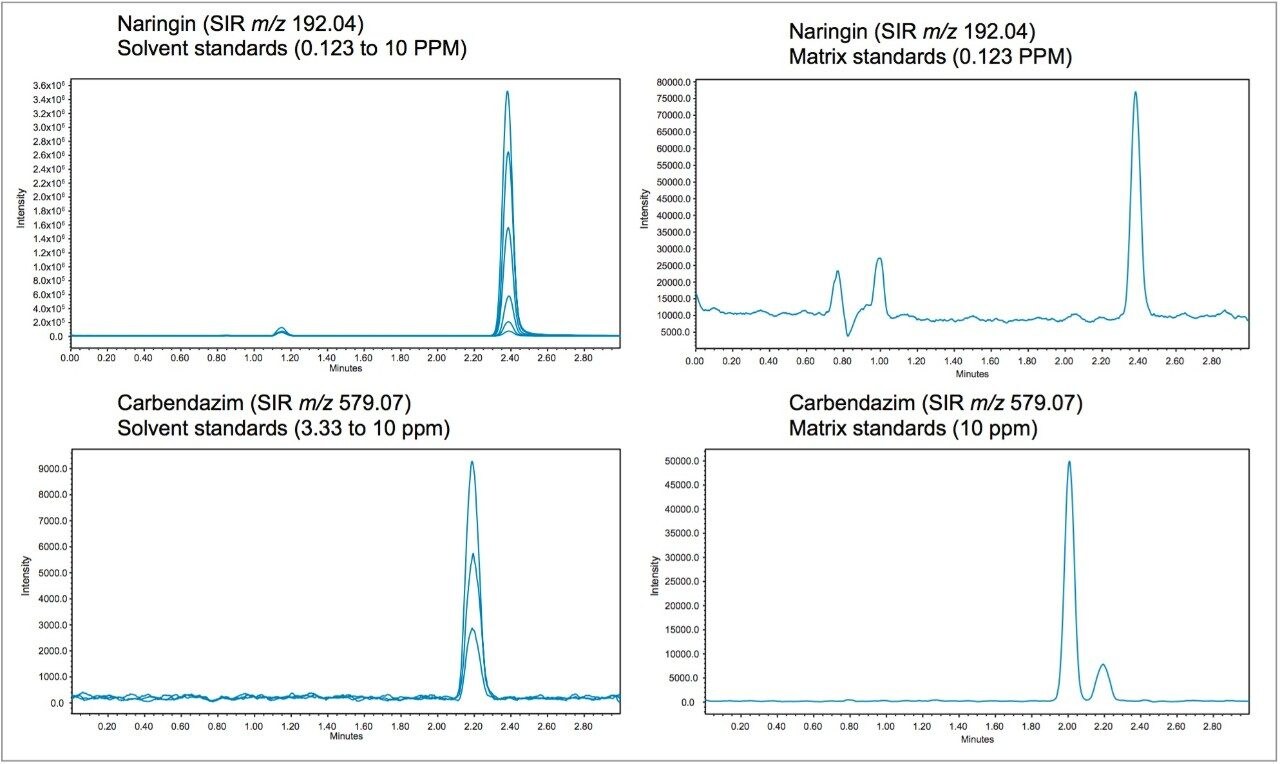
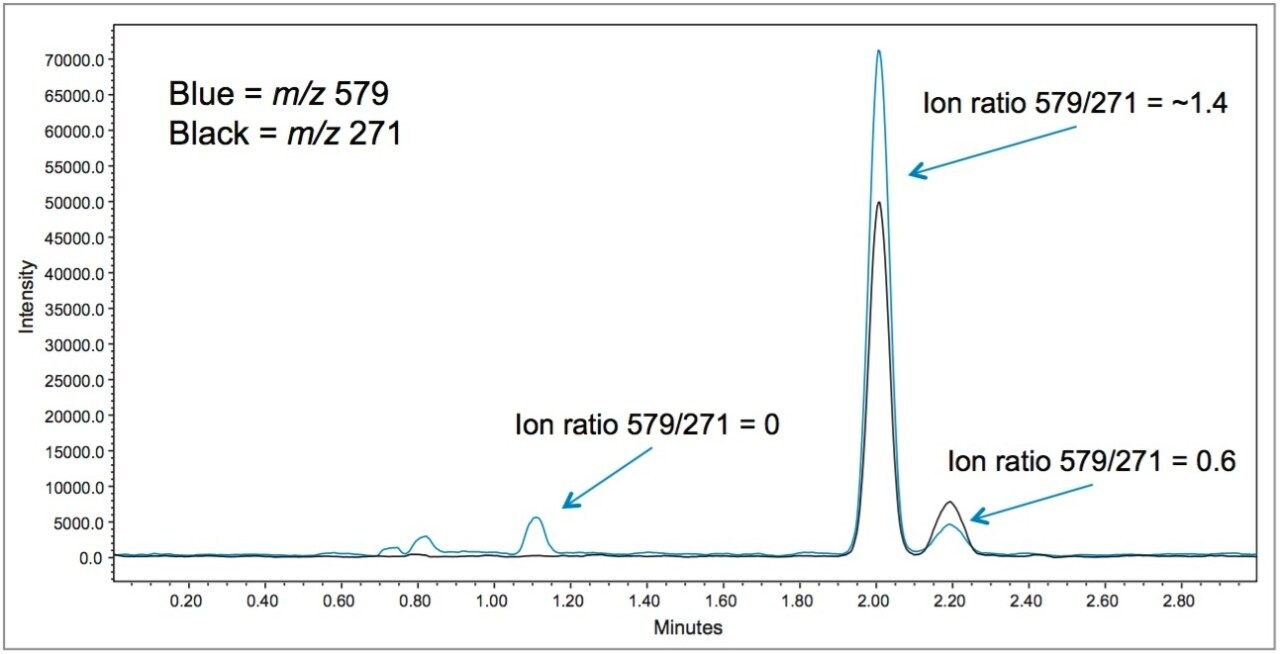
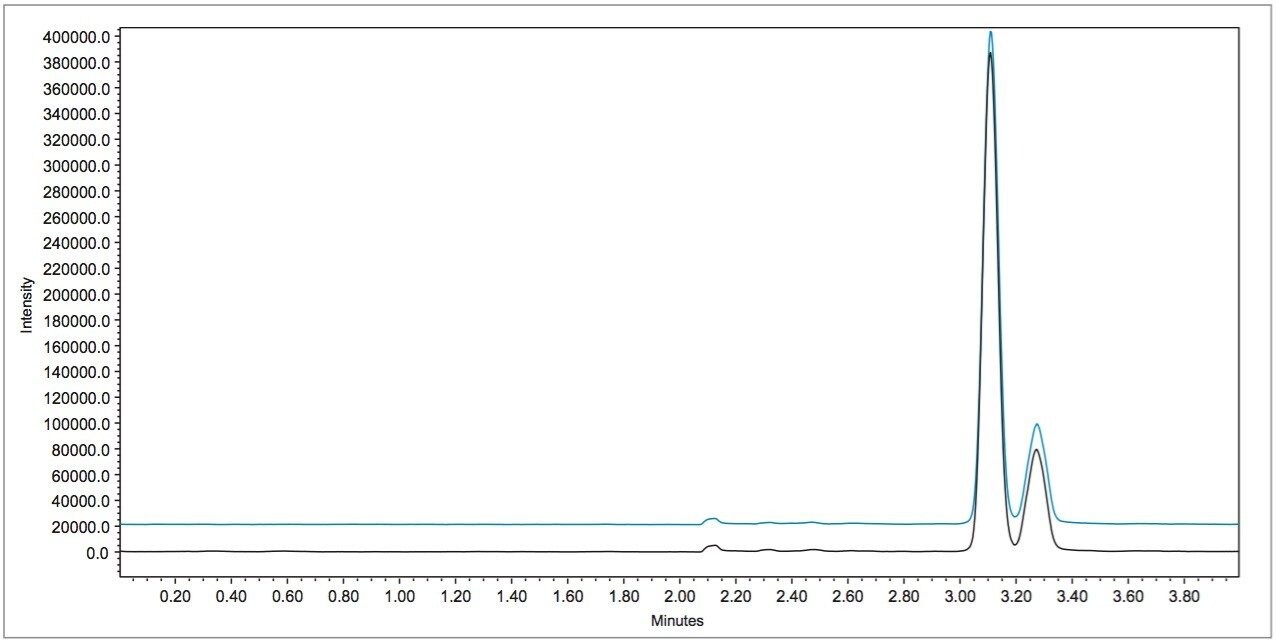
Mass detection of the solvent standards produced slightly different results than those observed for the matrix standards. For naringin solvent standards, a predominant peak was observed at 2.40 minutes; however, in the matrix standard, additional peaks were observed at 0.9 and 1.0 minutes. For carbendazim the differences were more pronounced. Specifically the SIR channel corresponding to the parent base ion for carbendazim (m/z 579.07) produced a single peak for the solvent standard, while an additional peak eluting just before the main peak was observed in the matrix standard (Figure 3).
Ion ratios can be used to confirm the identity of peaks by comparing the response from the parent ion to a fragment ion. As previously described, different SIR channels were collected using the ACQUITY QDa Mass Detector with a higher cone voltage used to induce fragmentation of the parent ion. Using these conditions, the ion ratios of 270.83 (fragment, CV 40) to 579.07 (parent, CV 10) for naringin was found to be 0.9 (Table 1). The ratio was repeatable at the 0.1 ppm limit for naringin and consistent across the dynamic range for solvent and matrix standards. For carbendazim this approach produced an ion ratio of 0.6 for 159.97 (fragment, CV 60) to 192.04 (parent, CV 25) (Table 1). This ratio was also repeatable at the 10 ppm limit for carbendazim and consistent across the dynamic range for solvent and matrix standards.
Given the consistency of the ion ratios these values can be used to confirm identification of the analytes in a complex matrix, where other analytes with the same m/z values may be present (Figure 3). For example, overlay of the parent and fragment SIR channels for carbendazim demonstrate the differences in the ion ratios for the various peaks observed in the matrix standard. As shown in Figure 4, the carbendazim peak at 2.2 minutes has a ratio of approximately 0.6, consistent with the ion ratio observed for the solvent standards. An additional interference peak at 2.0 minutes has a ratio of >1 and the peaks between 0.6 and 1.2 minutes have a ratio of ~0. These results provide confirmation that the peak at 2.2 minutes is carbendazim, and that the other predominant peaks are most likely components in the matrix. This approach allows for more reliable identification of known components in a complex matrix.
To increase sensitivity, a variety of larger injections of solvent and matrix standards were attempted. However, larger injections often lead to strong solvents effects and poor peak shape and decreased resolution, most notable in high organic diluents. As the diluent and the mobile phase matched, the effects of the increased injection volume were muted; however the resolution between naringin and carbendazim suffered and the peak tailing increased to unacceptable levels. When At-Column dilution (ACD) was deployed, larger injections (25 µL instead of 2 µL) gave acceptable resolution and peak tailing, which extended the sensitivity of each component.
The higher injection volumes were used to evaluate the dynamic range and sensitivity of the multi-dimensional approach using solvent standards with ACD and trap-and-elute. Calibration curves were produced over the range of 0.37 to 10 ppm for carbendazim (linear, R2 = 1.00) and from 0.0137 to 5 ppm for naringin (quadratic, R2 = 1.00) (Table 2, Figure 5). Precision was improved with this approach and was found to be 1.0% (solvent) and 0.6% (matrix) for naringin, and 1.6% (solvent) and 2.3% (matrix) for carbendazim. Recovery was 95.7% for naringin and 89.7% for carbendazim (Table 3). Ion ratios matched those produced during the original isocratic run.
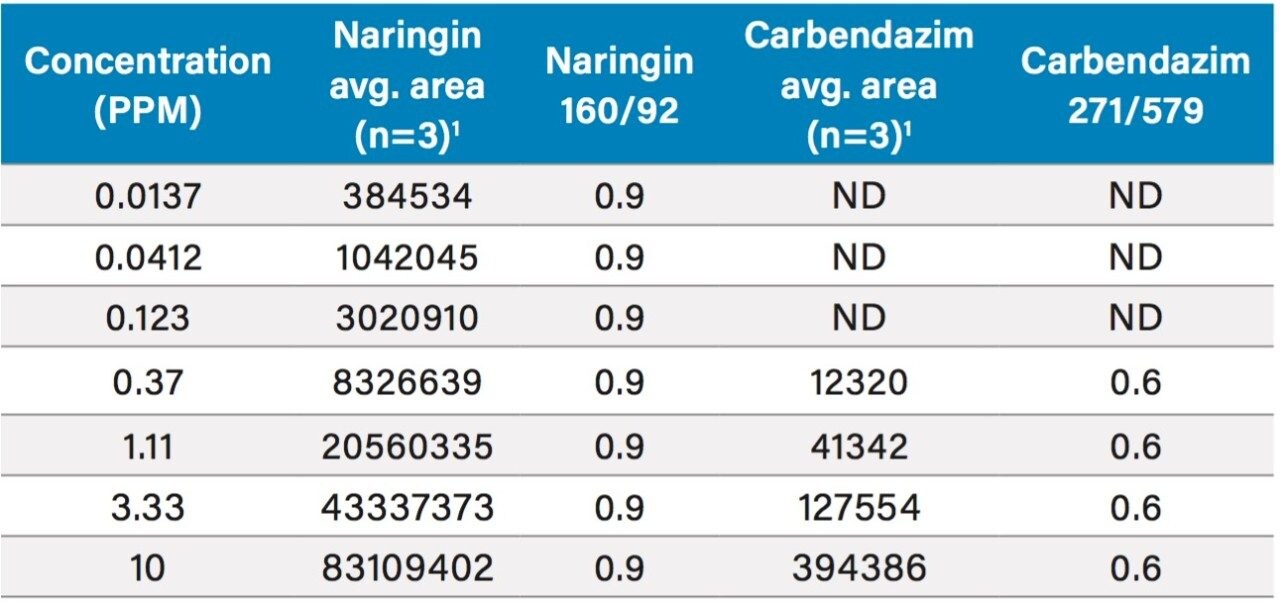
Table 2. Table of calibration data for solvent standards with ACD.
1Naringin area uses SIR m/z 192.04, Carbendazim data uses SIR m/z 579.07.
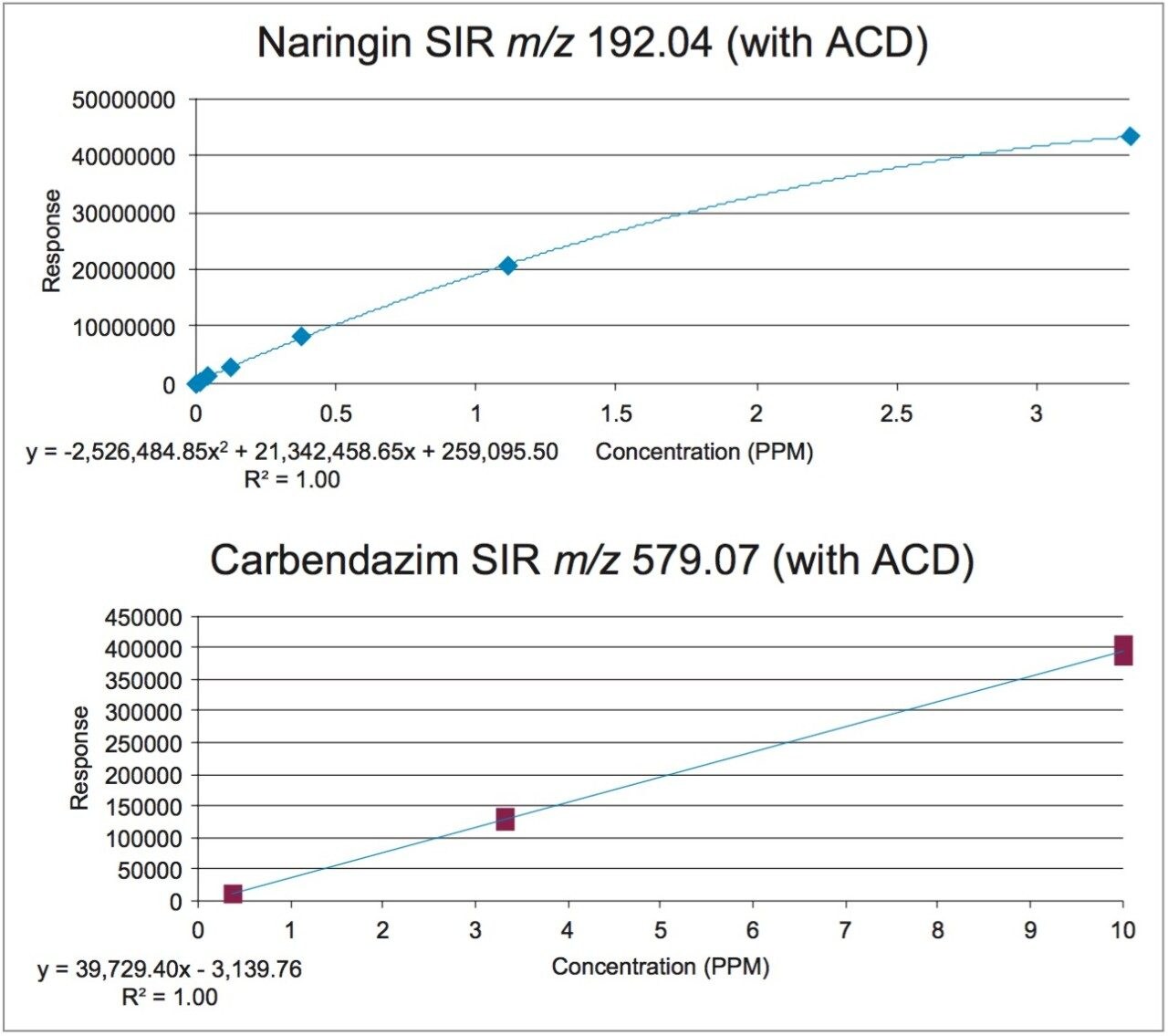
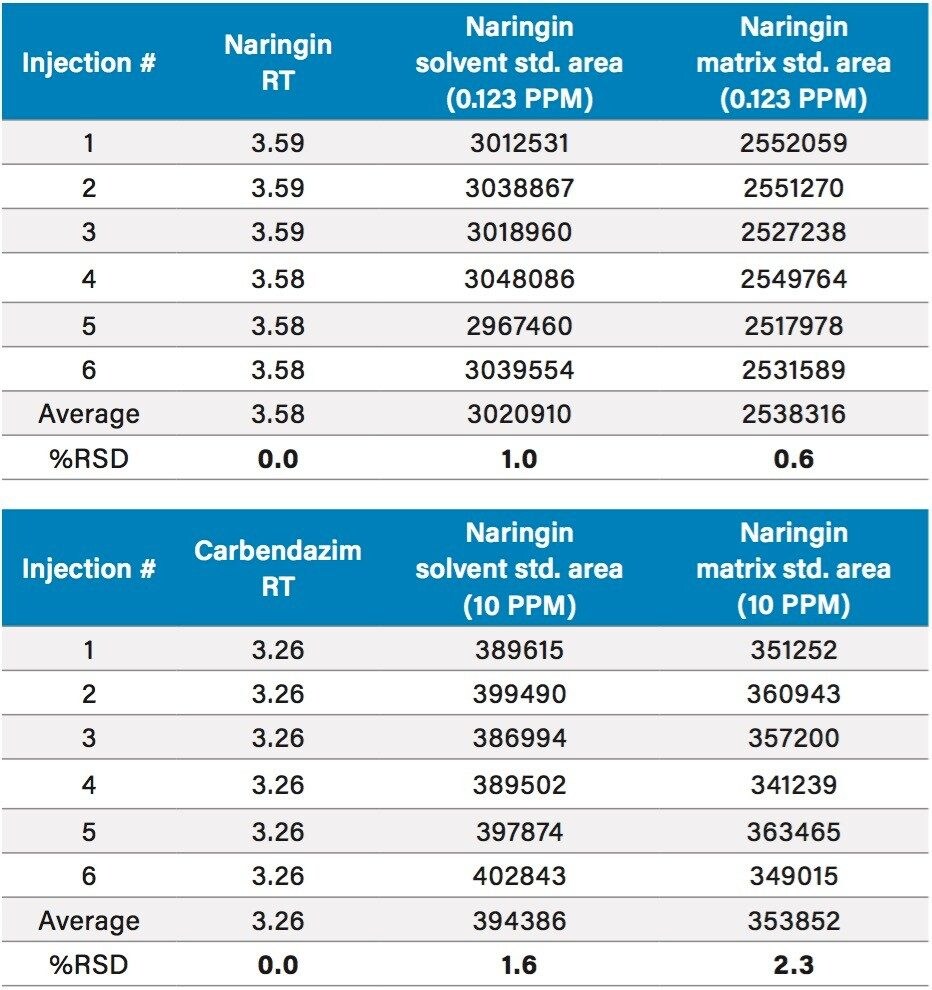
Table 3. Table of precision solvent and matrix standards with ACD.
1Naringin area uses SIR m/z 192.04, Carbendazim data uses SIR m/z 579.07.
Preparing samples in organic solvents is something that is appealing to food testing labs because higher organic diluents allow for reduced loss of material from poor solubility and interactions with glassware. ACD can allow injections of pure organic diluents because the dilution pump refocuses the material on the trap before eluting to the chromatographic column. Figure 7 shows an overlay of a 10 ppm matrix standard prepared in 60/40 water/MeOH with 10mM ammonium acetate and another prepared in 100% methanol. Regardless of the diluent, the same peak shape, resolution, and sensitivity is achieved.
An isocratic method has been developed for the analysis of naringin and carbendazim in orange juice. The ratio of parent and fragment ions were also used to confirm peak identity. Multi-dimensional techniques including at-column dilution (ACD) with trap-and-elute allowed the extension of the dynamic range to lower levels and improved precision. With at-column dilution, increased sensitivity was achieved by enabling the injection of larger volumes without loss of resolution or peak shape problems due to strong solvent effects. These results show how a greater dynamic range and improved precision can be achieved on a low cost mass detector in combination with ACD with trap-and-elute approach.
720005883, November 2017