This is an Application Brief and does not contain a detailed Experimental section.
This technology brief demonstrates the absolute quantitation of RapiFluor-MS labeled N-glycans using the RapiFluor-MS Quantitative Standard.
Together, the GlycoWorks RapiFluor-MS N-Glycan Kit and RapiFluor-MS Quantitative Glycan Standard provide a powerful new approach to quantitatively analyze N-glycans for proficiency testing, and to facilitate glycan-centric biomarker, as well as DMPK, studies.
Glycosylation plays an important role in the structure and function of proteins, and altered glycosylation is implicated in numerous diseases, such as cancer,1,2 autoimmune diseases,3 and neurodegenerative disorders.⁴ Furthermore, glycosylation is frequently determined to be a critical quality attribute for therapeutic glycoproteins.5,6 Just like characterization, quantitation of protein glycosylation is needed for various reasons, including the discovery of glycan biomarkers for diagnosis, prognosis, or risk prediction,⁷ and drug metabolism and pharmacokinetics (DMPK) studies.⁸ Quantitative measurements are also essential to assessing the proficiency of an analytical method, where they can be helpful in estimating yields, facilitating validation studies, and confirming the success of transferring a method to a different laboratory. Recently, a novel analytical approach was introduced for the preparation and analysis of N-glycans.
The strategy of this approach is based on combining the rapid release and labeling of N-glycans via the GlycoWorks RapiFluor-MS N-Glycan Kit with high-resolution hydrophilic interaction chromatography (HILIC) and sensitive fluorescence (FLR)-mass spectrometric (MS) analyses.⁹ In this work, we expand upon the capabilities of this approach and show how the RapiFluor-MS Quantitative Glycan Standard can be used to calibrate fluorescence response and thereby enable the absolute quantitation of RapiFluor-MS labeled N-glycans.
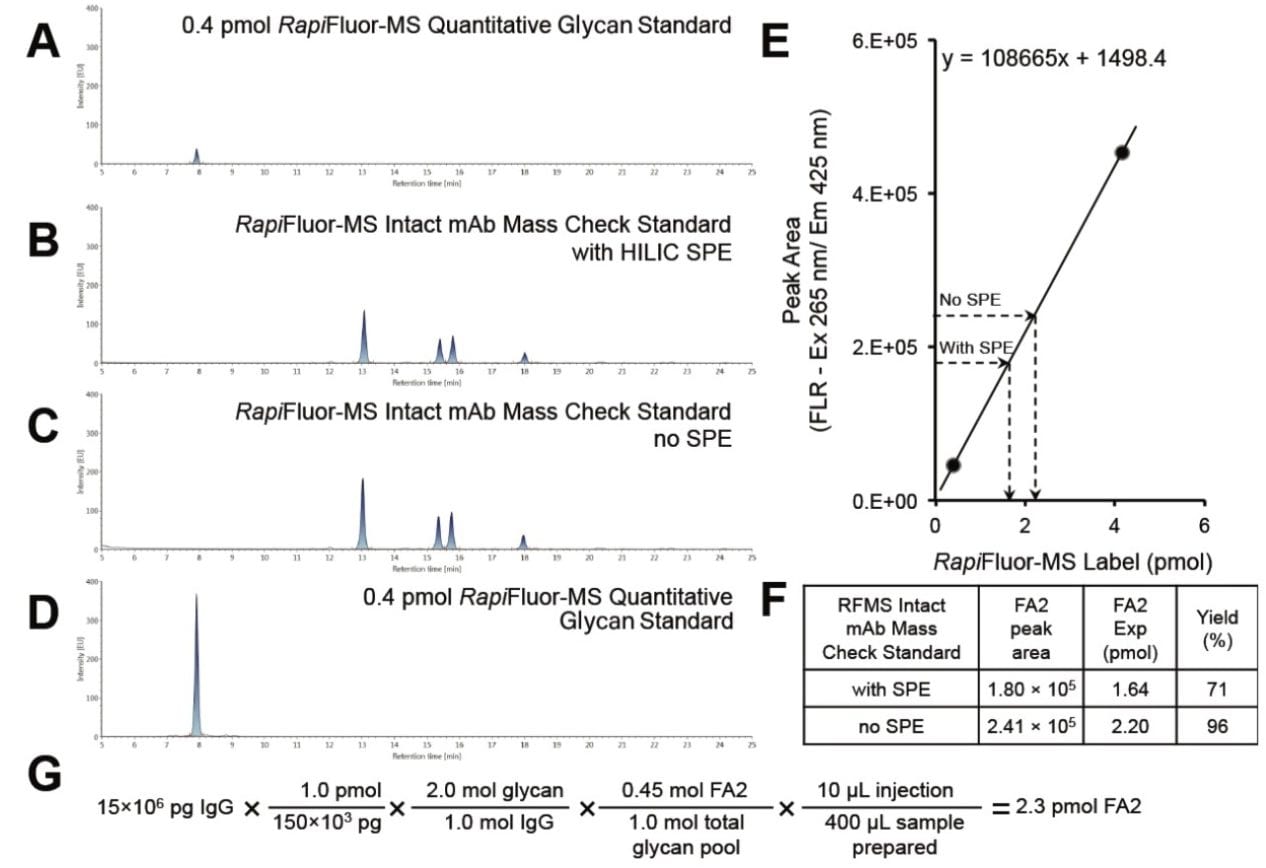
The RapiFluor-MS Quantitative Glycan Standard (p/n: 186008791) was purposefully designed to help analysts calibrate fluorescence responses observed during HILIC-FLR-MS separations of RapiFluor-MS labeled glycans. It is a synthetic peptide uniquely constructed of hydrophilic residues. Most importantly, it is derivatized by one mole equivalent of the RapiFluor-MS label and rigorously tested to ensure applicability to quantitative measurements. With a HILIC glucose unit (GU) value of approximately 4, it can be used as either an internal or external calibrant for N-glycan quantitation without interference issues from sample matrix void peaks or RapiFluor-MS labeled N-glycans, as demonstrated in Figure 1A–D. Since fluorescence response is linearly proportional to the amount of RapiFluor-MS label, fluorescence peak areas can be used to quantify specific RapiFluor-MS labeled glycans. For example, a linear regression can be graphed to relate observed fluorescence peak areas to different injected amounts of the RapiFluor-MS Quantitative Glycan Standard. Analyses have shown that a 4-point calibration curve consisting of 0.4, 0.8, 2.4, and 4.0 pmol of the standard provides an R² value of 1.0000. A quick 2-point (0.4 and 4.0 pmol) calibration curve for RapiFluor-MS labeled species can therefore be used to perform reliable absolute quantitation (Figure 1E).
Among the different reasons to perform a quantitative analysis, one is to check the proficiency of a GlycoWorks RapiFluor-MS N-Glycan Kit (p/n: 176003713) sample preparation. In such a case, a control standard, like the Intact mAb Mass Check Standard (p/n: 186006552), can be used to study proficiency and yields. Here, we have focused on the amounts of FA2 glycan obtained from the Intact mAb Mass Check Standard with and without HILIC solid phase extraction (SPE) cleanup, which we have calculated, from their measured fluorescence peak areas, to be 1.64 and 2.20 pmol (Figure 1F). Since 45% of the glycans released from the Intact mAb Mass Check Standard are known to correspond to the FA2 glycan, the yield of glycans released and labeled from the Intact mAb Mass Check Standard using the GlycoWorks RapiFluor-MS N-Glycan Kit with and without HILIC SPE cleanup can be calculated to be 71% and 96%, respectively (Figure 1F-G). As previously reported, this result confirms that a RapiFluor-MS N-glycan sample preparation is effectively lossless, save for the SPE step.⁹ The value of the SPE step should not, however, be discounted as its losses have been confirmed to be unbiased, and its use can improve the lifetime of the downstream HILIC column.
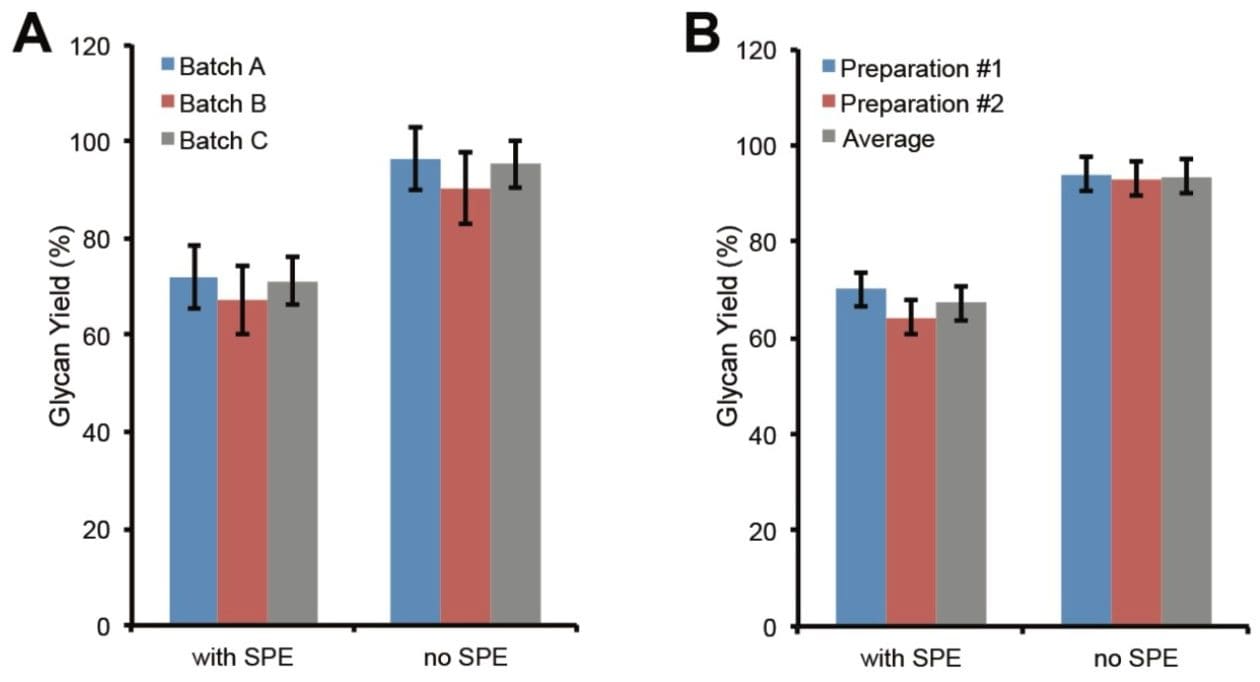
The above glycan yields were obtained with one vial of the RapiFluor-MS Quantitative Glycan Standard. With three different batches of RapiFluor-MS Quantitative Glycan Standard, the glycan yields of the same preparation of RapiFluor-MS labeled N-glycans ranged from 67% to 72% with HILIC SPE cleanup and 90% to 96% without SPE (Figure 2A). The displayed error bars correspond to the relative standard deviations of glycan yields obtained from three vials of RapiFluor-MS Quantitative Glycan Standard within the same batch. In sum, these findings demonstrate the noteworthy vial-to-vial and batch-to-batch reproducibility of the RapiFluor-MS Quantitative Glycan Standard. The average glycan yields obtained from the three batches of RapiFluor-MS Quantitative Glycan Standard were 70% with SPE and 94% without SPE (Figure 2B, preparation #1). In the same fashion, glycan quantitation was also performed on a second N-glycan preparation, and the yields were found to be 64% with SPE and 93% without SPE (Figure 2B, preparation #2). These data clearly demonstrate the high reproducibility of the GlycoWorks RapiFluor-MS N-Glycan Kit for glycan release and labeling. As they are, these results also provide evidence in support of the proficiency with which the GlycoWorks RapiFluor-MS N-Glycan Kit sample preparations were performed.
We have demonstrated the absolute quantitation of N-glycans through combining HILIC-FLR analyses with the use of the RapiFluor-MS Quantitative Glycan Standard. In this work, we defined an external calibration curve from the fluorescence responses obtained with two different injected amounts of the RapiFluor-MS Quantitative Glycan Standard. It was thereby possible to quantify the amounts of specific RapiFluor-MS labeled N-glycans. As applied to proficiency testing, use of the RapiFluor-MS Quantitative Glycan Standard made it possible to determine and compare the yields of two RapiFluor-MS N-glycan sample preparations. In turn, it was demonstrated that release, labeling, and SPE clean-up of glycans with the GlycoWorks RapiFluor-MS N-Glycan Kit is achieved with very high reproducibility. Together, the GlycoWorks RapiFluor-MS N-Glycan Kit and RapiFluor-MS Quantitative Glycan Standard provide a powerful new approach to quantitatively analyze N-glycans. Here, its utility for proficiency testing was highlighted, but it should also be noted that the RapiFluor-MS Quantitative Glycan Standard could be used to facilitate glycan-centric biomarker and DMPK studies.
720005981, April 2017