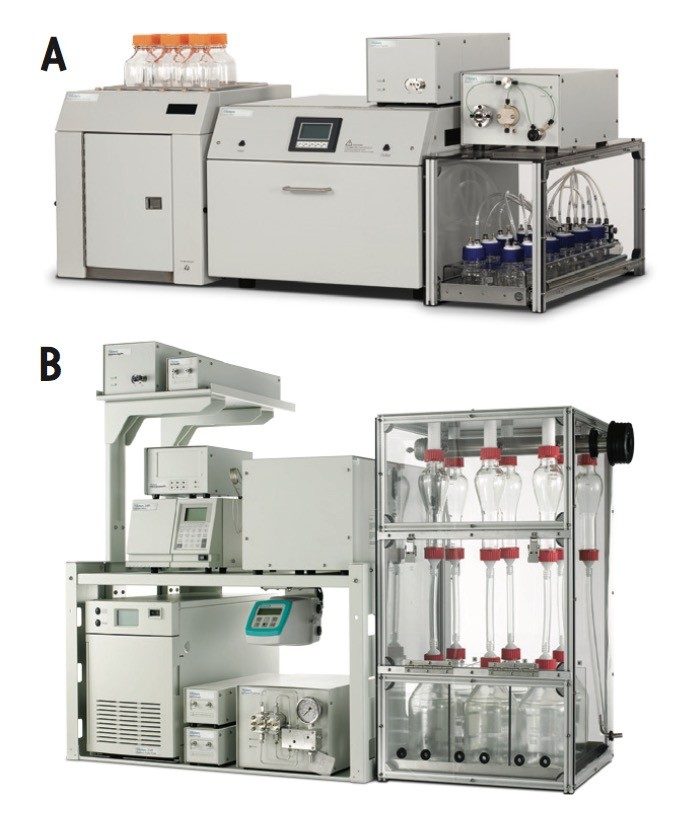
This application note demonstrates a complete SFE-SFC workflow solution using an MV-10 ASFE System (MV-10) and a Prep 80q SFC System for the extraction and purification of vanillin from whole vanilla beans. This process can be adapted to purify target compounds from a variety of natural products and matrices.
Nature is full of target compounds that are used in a variety of consumer products from pharmaceuticals and nutraceuticals to flavors and fragrances. Currently, natural flavors are in high demand, a trend that ties in with increased consumer awareness and preference for traditional or organic food.1 Vanilla is the world’s most popular flavor, used in the production of ice-cream, yogurt, beverages, baked goods, cereals, and even chocolate. Vanillin is the primary flavor component that lends that signature vanilla flavor and is present in varying amounts depending on the origin and treatment of the beans.2 Natural vanilla extracts, based on their concentration level, cost around $1800 per kg (~$800 per lb), while synthetic vanillin costs $25 per kg (~$10 per lb).1
Currently, the two most widely used techniques for vanilla bean extraction are percolation (with ethanol and water) which can take 2–3 days, and the oleoresin method that uses ethanol and requires 8–9 days. Extractions using supercritical fluid CO2 have been utilized as well; this process produces better quality extracts in less time that have a higher vanillin concentration and therefore demand higher prices.3,4
In a supercritical fluid workflow, CO2 with or without the addition of an organic modifier is used to extract (SFE) and purify (SFC) target compounds. The CO2 used as a solvent is safe and the extracts produced by this process are free from biological contaminants, have longer shelf life, high potency, and address major international concerns regarding residual solvent concentration.3 Here we demonstrate a complete SFE-SFC workflow solution using an MV-10 ASFE System (MV-10) and a Prep 80q SFC System (Figure 1) for the extraction and purification of vanillin from whole vanilla beans. This process can be adapted to purify target compounds from a variety of natural products and matrices
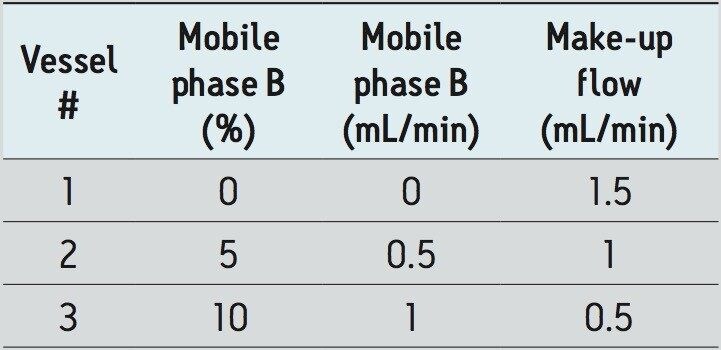
Whole Mexican vanilla beans were obtained from a local supermarket. Initially, three SFE solvent conditions were evaluated using three 5 mL extraction vessels each containing approximately 2 grams of diced Mexican vanilla beans. By adjusting the make-up flow accordingly, the total ethanol flow rate was kept at 1.5 mL/min into the collection bottles. These conditions are shown in Table 1. The extracts were diluted to 100 mL for evaluation. Only relative yields were determined, no attempt was made to exhaustively extract the vanillin. To obtain pure vanillin, the Prep 80q SFC System was utilized to separate and collect the vanillin from the extract.
All supporting analysis was performed on an ACQUITY UPC2 System. To determine extract and fraction yields, a linear calibration curve (R2=0.9999) was developed on the ACQUITY UPC2 System using a vanillin standard at concentrations from 0.01 to 0.5 mg/mL. To determine fraction recovery on the Prep 80q SFC, an injection standard was prepared by doing five 2 mL injections and collecting the flow directly off of the injector. Both the standard and the fraction were diluted to 50 mL for analysis.
|
System: |
MV-10 ASFE System |
|
Software: |
ChromScope v1.5 |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
Ethanol (200 Proof HPLC Grade) |
|
Flow rate: |
10 mL/min |
|
Make-up solvent: |
Ethanol (200 Proof HPLC Grade) |
|
Pressure: |
300 Bar |
|
Temp.: |
40 °C |
|
Method steps: |
Dynamic 1: 3 min
Static: 60 min
Dynamic 2: 30 min
|
System: |
Prep 80q SFC System with a 2489 UV/Vis Detector |
|
Software: |
ChromScope v1.2.1 |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
Ethanol (Reagent HPLC Grade) |
|
Flow rate: |
72 mL/min |
|
Gradient: |
5 to 15% B in 4.5min |
|
Pressure: |
220 Bar |
|
Temp.: |
40 °C |
|
UV: |
267nm |
|
Preparative column: |
Viridis 2-EP Column (19 x 150mm, 5 μm) |
|
Injection volume: |
2 mL |
|
System: |
ACQUITY UPC2 System |
|
Software: |
MassLynx v4.1 |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
Ethanol (200 proof HPLC grade) |
|
Flow rate: |
1.5 mL/min |
|
Gradient: |
2 to 20% in 5 min |
|
Pressure: |
150 bar |
|
Temp.: |
40 °C |
|
Analytical column: |
ACQUITY UPC2 BEH 2-EP Column (3 x 100 mm, 1.7 μm) |
|
Injection volume: |
2 μL |
|
Scan: |
220–400nm |
|
Absorbance compensated: |
267 nm |
|
Reference: |
320–400nm |
There are two main factors to consider in SFE method development – yield and extract complexity. Depending on the application, overall yield is important, but the complexity of the extract can also be important to the purification process. If an extract has a higher percentage of the compound of interest (even if the yield is relatively lower) and fewer impurities, it simplifies and improves efficiency in the collection process. If possible, CO2-only conditions are preferred for food related applications because of improved consumer safety and a lack of organic solvent waste.
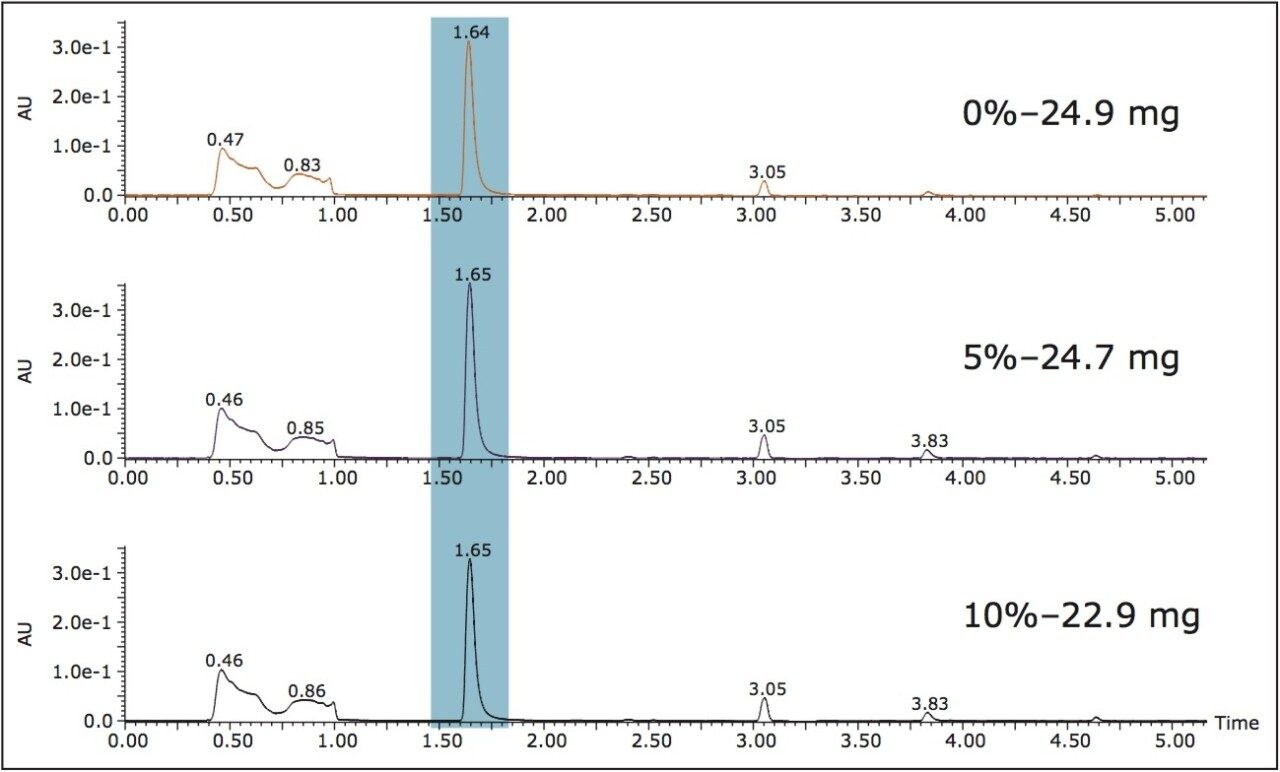
Using the Mexican vanilla beans, SFE method development was performed using the automated capabilities of the MV-10 ASFE System. The software controlled automation allows the user to program various extraction conditions for multiple samples and run them unattended. Extractions of three samples were performed at 0%, 5%, and 10% ethanol. Chromatographic analysis of the resulting extracts and their yields are shown in Figure 2. The average yield was 24.2 mg, which represents 1.2% of the approximately 2 g of initial sample. There was little statistical difference in the extraction yields and complexities. Since a CO2-only method is preferred, the 0% extract was selected for purification.
Generally when purification is the goal, it is more practical for method development to be performed at the analytical scale to save solvent, time, and sample. The initial gradient on the ACQUITY UPC2 System was 2–20% ethanol in 5 minutes (1.44%/cv), and the vanillin eluted at about 6%. In order to optimize the separation for purification, the gradient was modified and a loading study was performed (Figure 3). The modified gradient was 5–15% ethanol in 3 minutes (1.33%/cv). Under these conditions, separation was maintained up to 10 μL while reducing the overall run time.
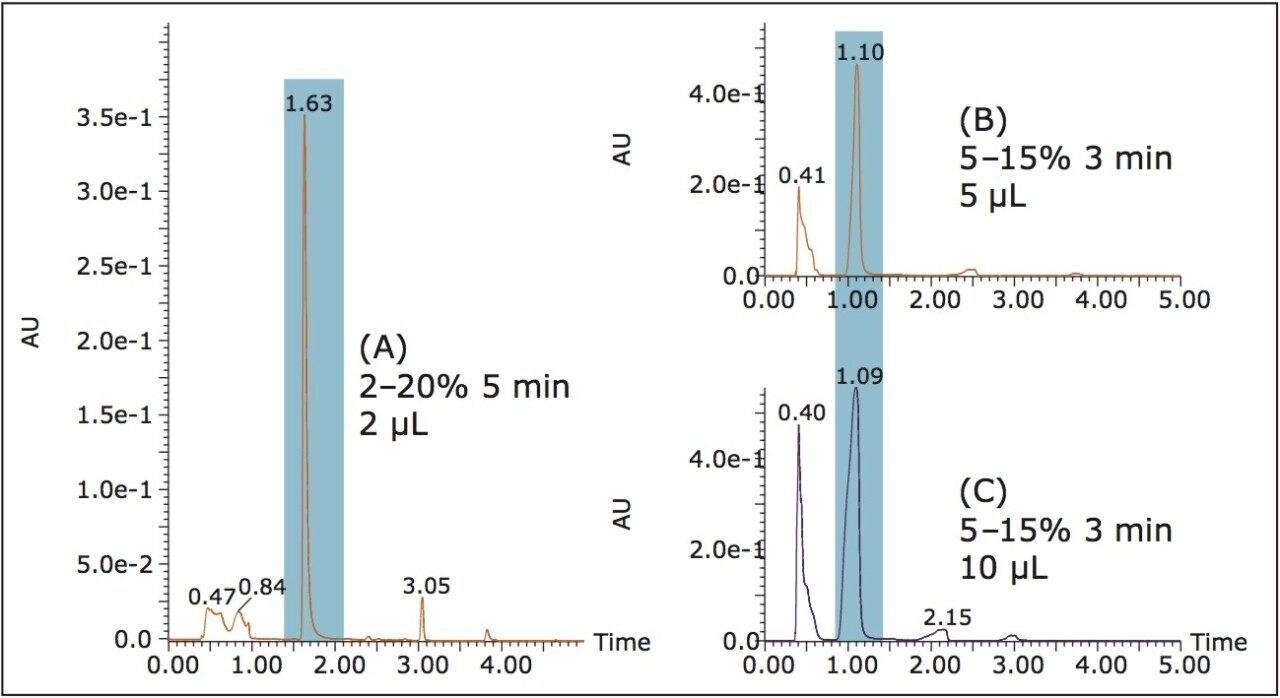
The optimized UPC2 method parameters were scaled for the 19 x 150 mm Viridis 2-EP Prep Column, resulting in a 5–15% 4.5 min gradient. Due to the small particle size of the column and tubing I.D. on the ACQUITY UPC2 System, the front pressure was 260 bar (110 bar pressure drop across the system). In order to ensure consistent separation, the two systems needed to be operated at similar pressures. To maintain a 260 bar front pressure on the Prep 80q SFC System, the BPR pressure was set to 220 bar (40 bar pressure drop). The calculated geometric scale-up was 600 μL, but the experimental separation allowed for much higher loading, so 2 mL injections were used (Figure 4).
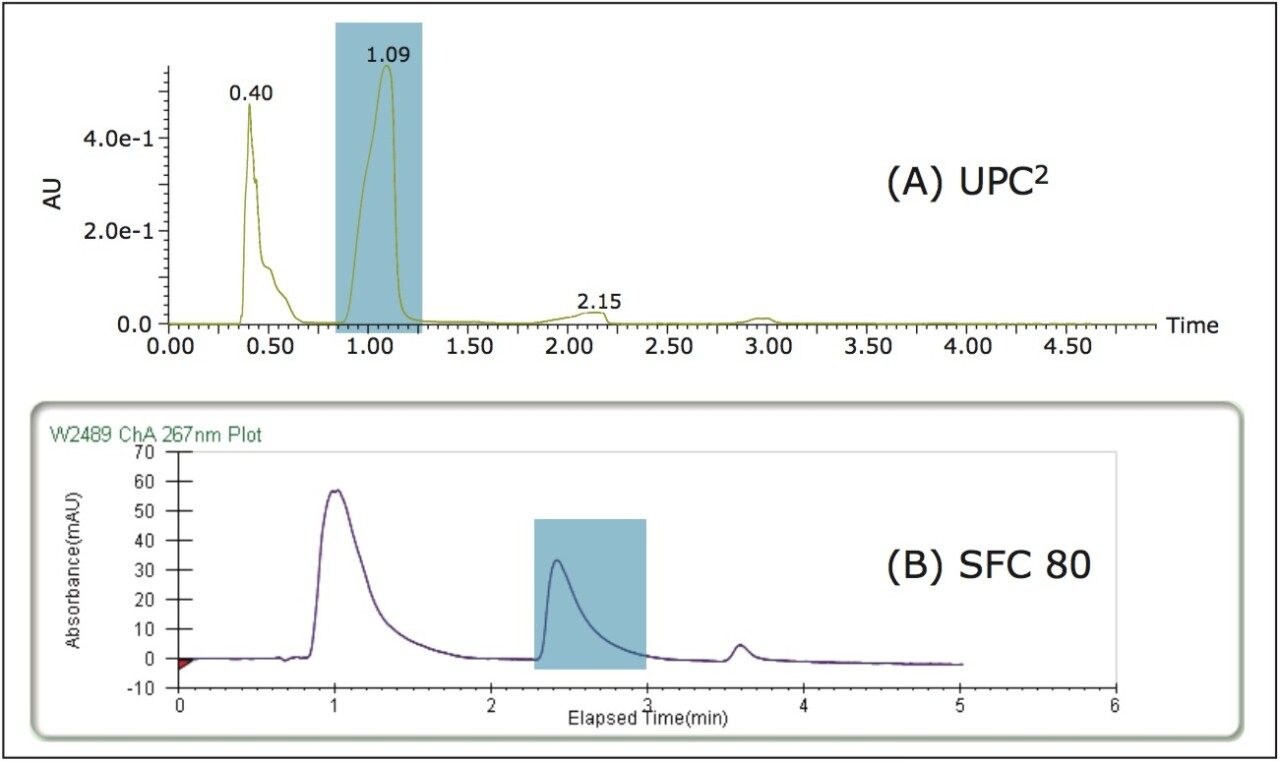
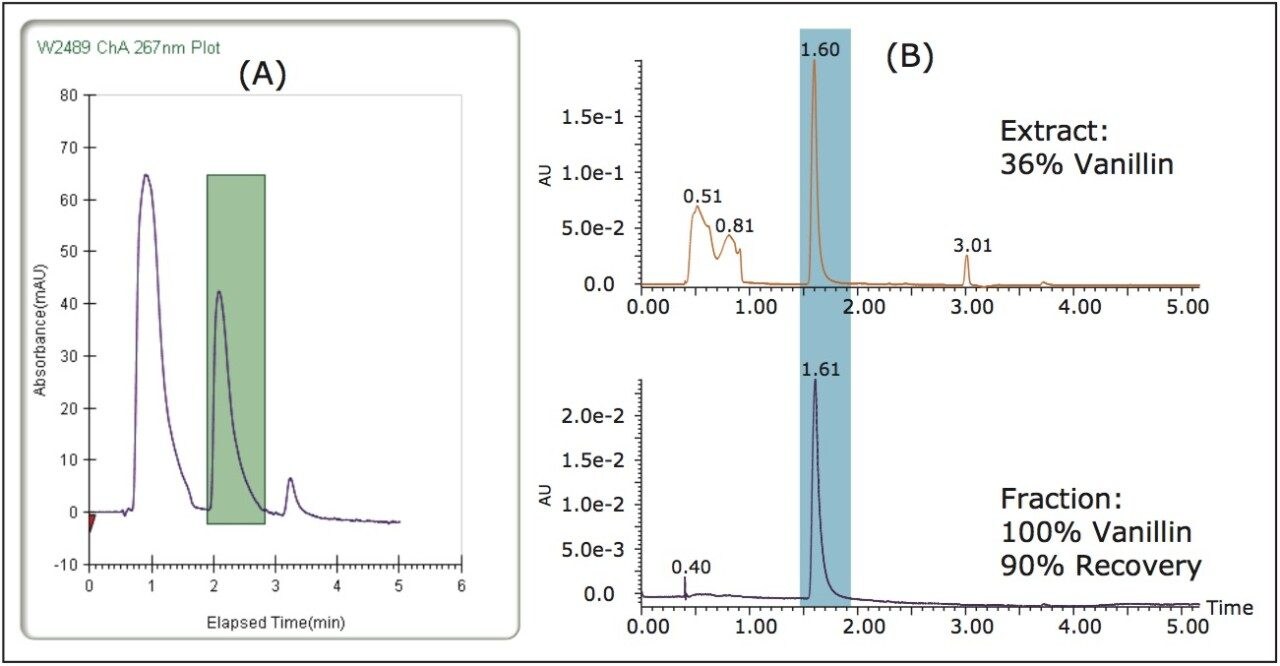
The Prep 80q SFC System is designed for bulk purification, making it useful for applications where large amounts of a single sample need to be purified. While various collection methods can be used on the Prep 80q SFC System, in this case fractions were collected by time. The SFC 80 vanillin fraction was evaluated for purity and recovery on the ACQUITY UPC2 System. Figure 5 shows collection on the SFC 80 along with analysis of the collected fraction on the UPC.2 The initial extract was only 36% vanillin based on the peak area counts in the UV chromatogram, after purification on the SFC 80 the fraction was close to 100% pure vanillin (no impurities were detected). Recovery of the vanillin in the extract was greater than 90%.
720005457, July 2015