This is an Application Brief and does not contain a detailed Experimental section.
This application brief eliminate carryover from sample to sample to ensure accurate quantification of unknown dioxin and furan containing samples.
Carryover was significantly reduced to 0.007% which is a 20-fold decrease compared to the original values. This virtually eliminated the chance of a false positive result.
Dioxins and furans are produced when organic compounds are incinerated in the presence of chlorine for example during PVC production, paper bleaching, and from natural sources such as volcanoes. Dioxins are extremely toxic and readily bioaccumulate in many animal species due to their lipophilic properties. They are also suspected mutagens and carcinogens. They are also suspected mutagens and carcinogens.
The Xevo TQ-S with Atmospheric Pressure GC (APGC) has provided a very sensitive detection system for the accurate determination of dioxins and furans at regulatory levels. During the analysis of samples of an unknown concentration, extremely high levels of these compounds may be observed. Therefore there is the potential for carryover of target compounds into the following sample injection.The consequence of this would be a falsely elevated quantitative result.
The carryover was investigated on an APGC fitted with a split/splitless injector coupled with aWaters Xevo TQ-S. A single goose-neck splitless liner was installed into the injector. Five nonane washes were performed pre and post injection. A 1 in 10 dilution of the CS 5 standard from Wellington Laboratories (Guelph, ON) was injected followed by several nonane blanks.
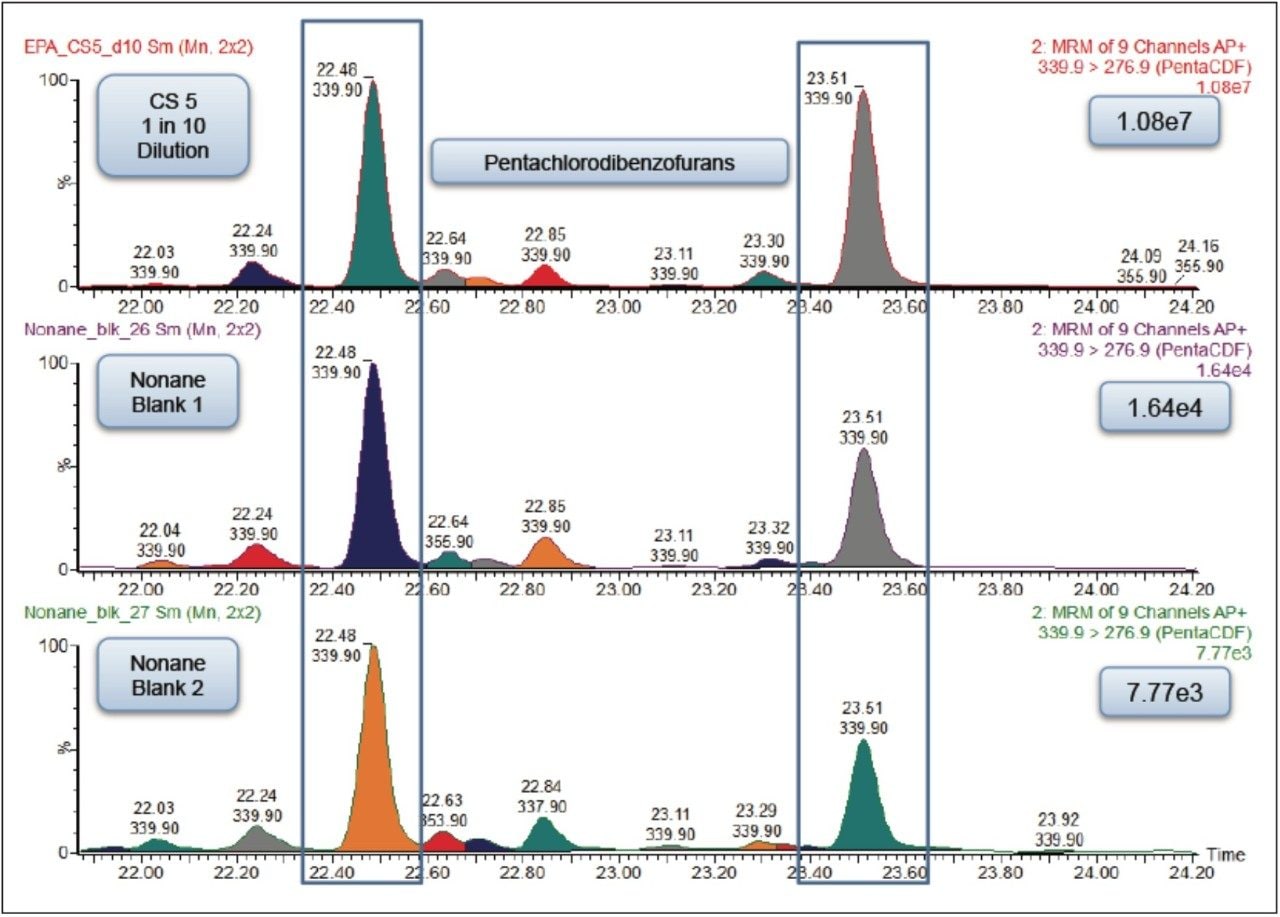
The initial carryover, calculated using peak height, was 0.15% (Figure 2). This level of carryover could cause inaccurate quantification in samples analyzed immediately following this in a batch analysis. Experiments were performed in order to reduce the carryover observed to an acceptable level.
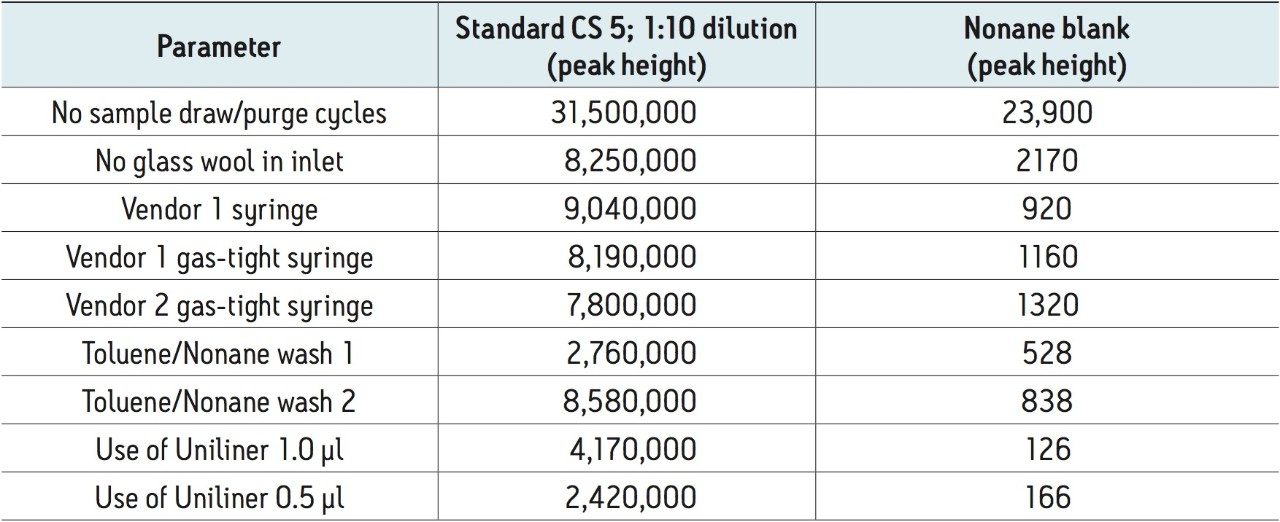
A number of possible approaches to reduce carryover were investigated:
Each of these approaches were tested to see how they affected the carryover from the 1 in 10 dilution of the CS 5 Standard to the response seen from the nonane blank injected immediately afterwards. The largest reduction in carryover was obtained by changing the wash solvents to toluene and nonane combined with the use of a Restek Uniliner (Table 1). By combining both of these changes, the carryover was significantly reduced to 0.007% which was a 20-fold decrease compared to the original values (Figure 3). This virtually eliminated the chance of a false positive result.
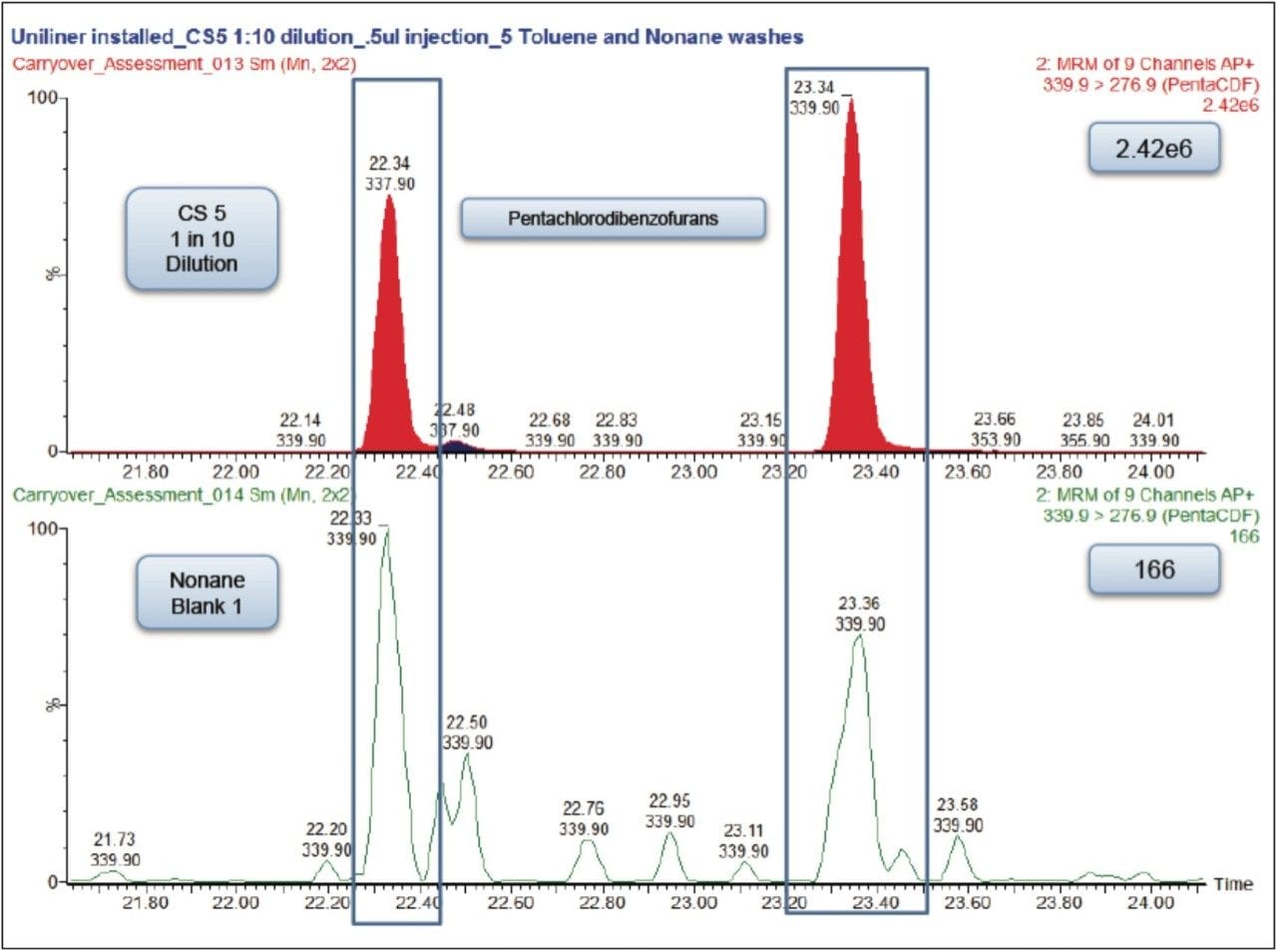
In order to ensure accurate quantification during dioxins and furans analysis on the Xevo TQ-S with APGC, the correct solvent washes and inlet liner should be used. Five washes of toluene followed by five washes of the sample diluent pre- and post-injection should be performed. Also a Uniliner should be installed into the inlet. The Sky 4.0 mm ID Drilled Uniliner Inlet Liner with Hole near Top from RESTEK (Part number 23311.1) was used successfully in the tests described here. The Uniliner design minimizes active sites in the sample pathway and reduces injection port discrimination. The analytical column connects directly to the bottom of the Uniliner via a press-fit seal, eliminating sample contact with any part of the injector below the column inlet and thus minimizing carryover.
By performing the analysis with this recommended configuration, confident and accurate analysis of dioxins and furans can be performed at low detection limits.
720004964, March 2014