
This application note describes the use of Waters ACQUITY UPLC H-Class coupled with the SQ Detector 2 for the rapid analysis of PAA in ink.
This application provides improved confidence in the identification and quantification of Primary Aromatic Amines (PAAs) offering:
PAAs are widely used in high amounts as a chemical feed stock within the chemical industry, and many of them are highly toxic to humans.1,2,3 PAAs can be used to produce many commodities, such as pharmaceuticals, pesticides, explosives, epoxy polymers, rubber, aromatic polyurethane products, and azo-dyes. They can be found in final products due to incomplete reactions, as impurities, by-products, or as degradation products. PAAs can be produced as by-products of azo dyes, which are a diverse and widely used group of organic dyes. Azo dyes have a wide range of uses including special paints, printing inks, varnishes, and adhesives; and can be found in many products such as textiles, cosmetics, plastics, and also in food contact material.
The inks and dyes industry is highly legislated and manufacturers that use these materials must monitor and quantify various regulated parameters, such as the presence or absence of PAAs.
Previous example methodologies for the analysis of PAAs include: GC-MS analysis following ion-pair extraction with bis-2-ethyl phosphate followed by derivatization with isobutyl chloroformate;4,5 UPLC analysis following a solid phase extraction (SPE) using cation-exchange cartridges;6 and reduction by liquid phase sorbent trapping followed by thermal desorption GC-MS analysis.7 Many previously used methods for PAA analysis lack robustness, selectivity, and sensitivity, and require lengthy, costly and time-consuming pre-treatments (derivatization, SPE).
Many PAAs have either a proven or suspected carcinogenic nature and are highly toxic, so there are a range of potential health risks that have led to strict worldwide regulations. U.S. FDA regulations (21 CFR 74.705 and 21 CFR 74.706) restrict the use of azo dyes that could degrade to PAAs; whereas EU regulations (commission directive 2002/72/EC and the amendment 2007/19/EC) set legislative limits for the release of total PAAs from food contact material.
Analytical laboratories require accurate and robust techniques to ensure confidence and versatility in meeting these legislative requirements The SQ Detector 2 offers a flexible solution for the ink and dyes industry.
This application note describes the use of Waters ACQUITY UPLC H-Class coupled with the SQ Detector 2 for the rapid analysis of PAAs in ink.
|
LC system: |
ACQUITY UPLC H-Class |
|
Runtime: |
10.00 min |
|
Column: |
ACQUITY UPLC BEH C18, 1.7 mm, 2.1 x 50 mm |
|
Column temp.: |
40 °C |
|
Mobile phase A: |
10 mL of 1 M aqueous ammonium acetate solution and 990 mL water |
|
Mobile phase B: |
10 mL of 1 M aqueous ammonium acetate solution and 990 mL methanol |
|
Flow rate: |
0.5 mL/min |
|
Injection volume: |
10.0 μl |
|
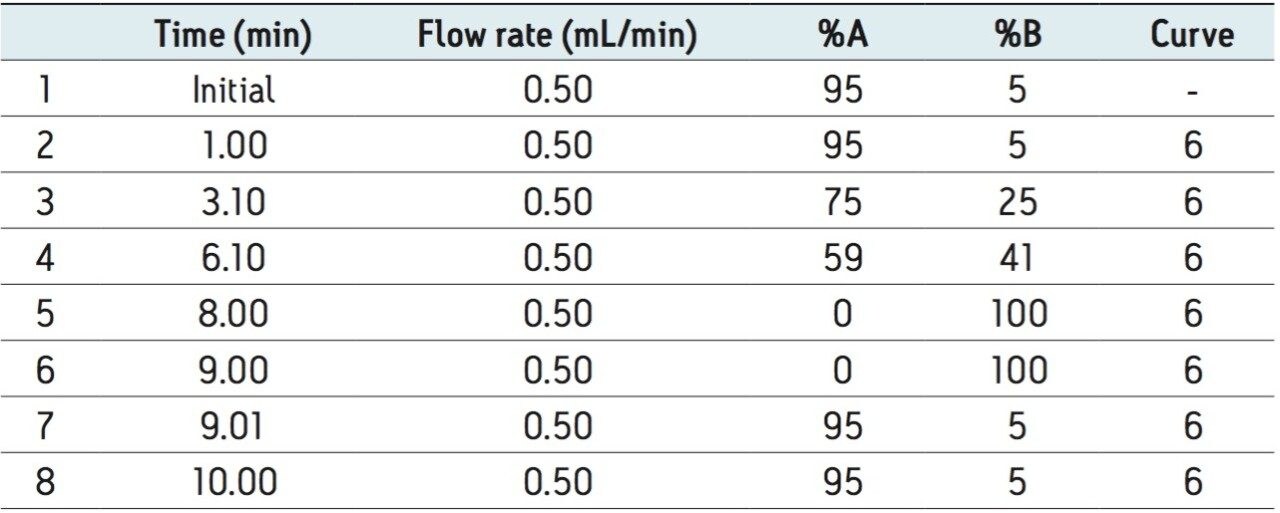
Mobile phase gradient is detailed in Table 1. |
|
MS system: |
SQ Detector 2 |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
3.0 kV |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
350 °C |
|
Desolvation gas: |
650 L/hr |
|
Cone gas: |
20 L/hr |
|
Acquisition: |
Selected Ion Recording (SIR) |
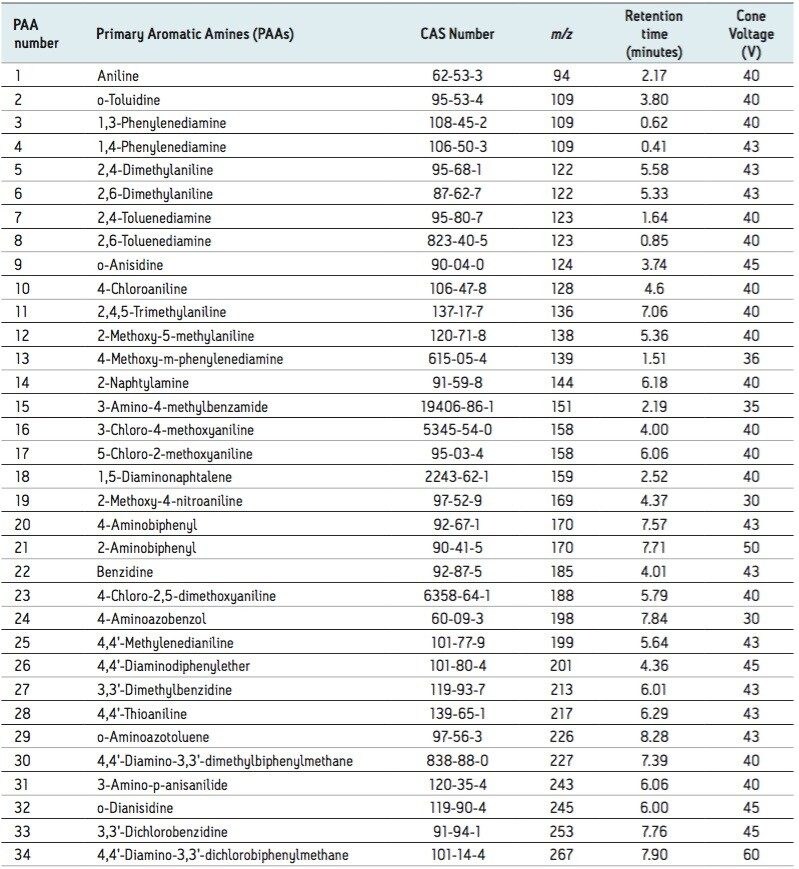
Variables such as cone voltages, desolvation gas (temperature and flow rate), and cone gas flow rate were optimized using solvent standards. The list of PAAs, associated CAS number, expected retention times, and cone voltages are detailed in Table 2. The established SIR MS method is illustrated in Figure 1.

MassLynx Software v.4.1 was used to control the ACQUITY UPLC H-Class and the SQ Detector 2 and also for data acquisition. Data quantitation was achieved using TargetLynx Application Manager.
Many gains can be accomplished using an ACQUITY UPLC System for chromatographic separation, due to the reduced column particle size (sub-2 μm), which results in improvements in speed and peak capacity, with superior sensitivity and resolution efficiently achievable over HPLC analysis.
During method development, considerations need to be given to the appropriate detector to use in order to meet the analytical requirements. The use of mass spectral detection over core detectors (e.g. UV or fluorescence) offers advantages in areas such as sensitivity and selectivity, especially where complex matrices are present. Matrix effects can be greatly reduced by using mass spectral detection over DAD (UV) detection and this can be demonstrated by considering many of the PAAs detailed within this application. Examples can be seen considering the PAAs, 2-Aminobiphenyl and 3,3'-Dichlorobenzidine. When using the current UPLC conditions the two compounds are not completely resolved giving retention times of 7.71 and 7.76 minutes respectively. Using mass spectral detection, the resulting efficient selectivity is illustrated in Figure 2.
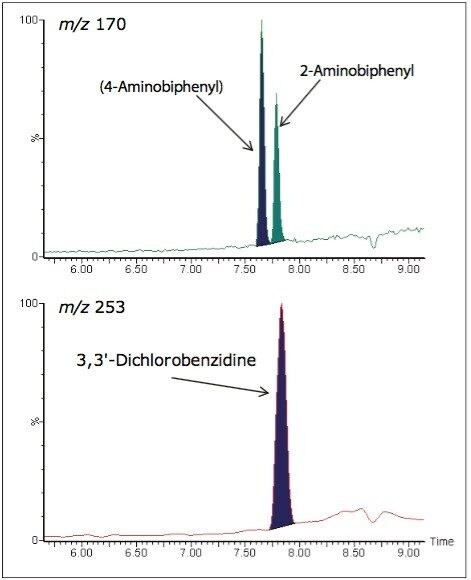
In this example when using UV detection due to the UV absorbing nature of the solvents used, the ink matrix, and other PAAs present this level of selectivity is very hard to achieve. This reduced selectivity can be demonstrated by again considering the PAAs, 2-Aminobiphenyl and 3,3'-Dichlorobenzidine in solvent standards. When considering individual solvent standards for 2-Aminobiphenyl and 3,3' Dichlorobenzidine, maximum UV absorbance can be found at 295 and 284 nm respectively. When comparing individual solvent standards against mixed solvent standards, the reduction in selectivity is demonstrated in Figures 3a and 3b, which could potentially lead to misidentification, poor integration, and false positive results.
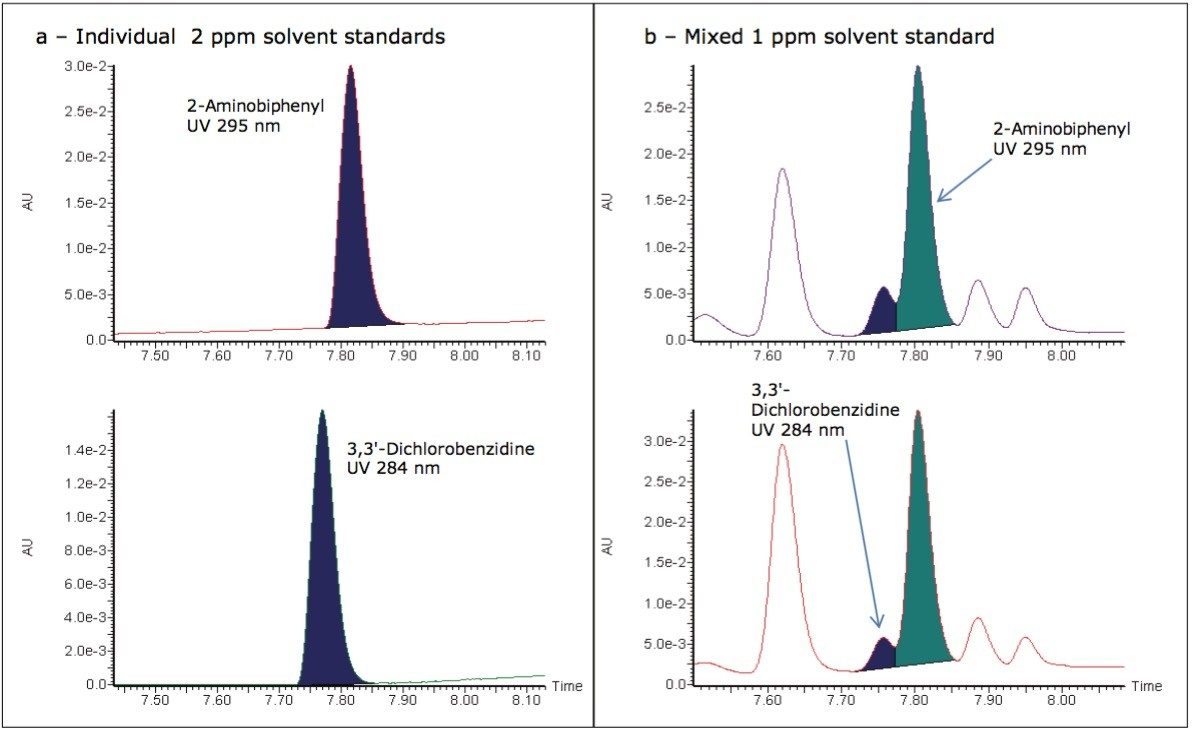
Figure 3. a) UV chromatograms for 2-Aminobiphenyl and 3,3' Dichlorobenzidine in individual solvent standards;
b) UV chromatograms for 2-Aminobiphenyl and 3,3'-Dichlorobenzidine in a mixed solvent standard.
Improvements in selectivity in this example could only be made by changing the chromatographic separation by altering the UPLC conditions to reducing the solvent gradient, which would result in longer run times and associated increases in solvent usage.
The analysis of 34 PAAs was achieved using Waters SQ Detector 2 with an electrospray ionization (ESI) source, coupled to an ACQUITY UPLC H-Class System in SIR mode.
Optimum UPLC and SIR conditions were developed, with the elution of all compounds within a 10-minute run.
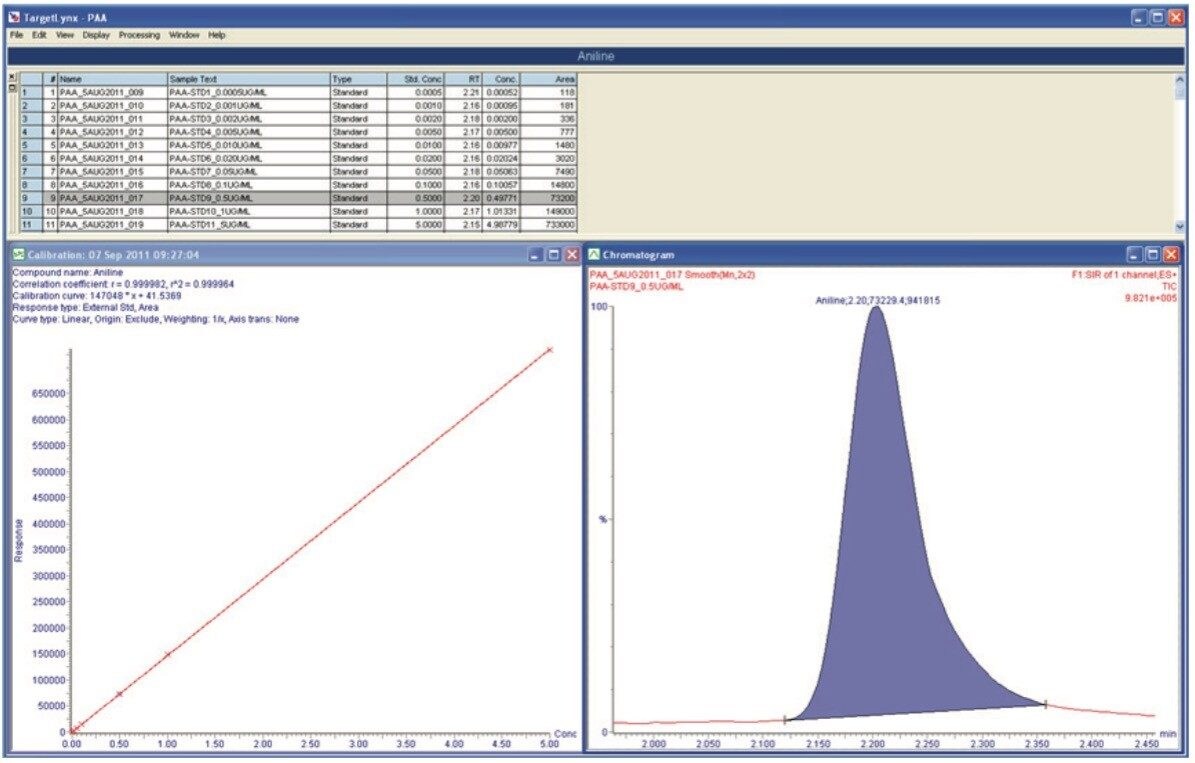
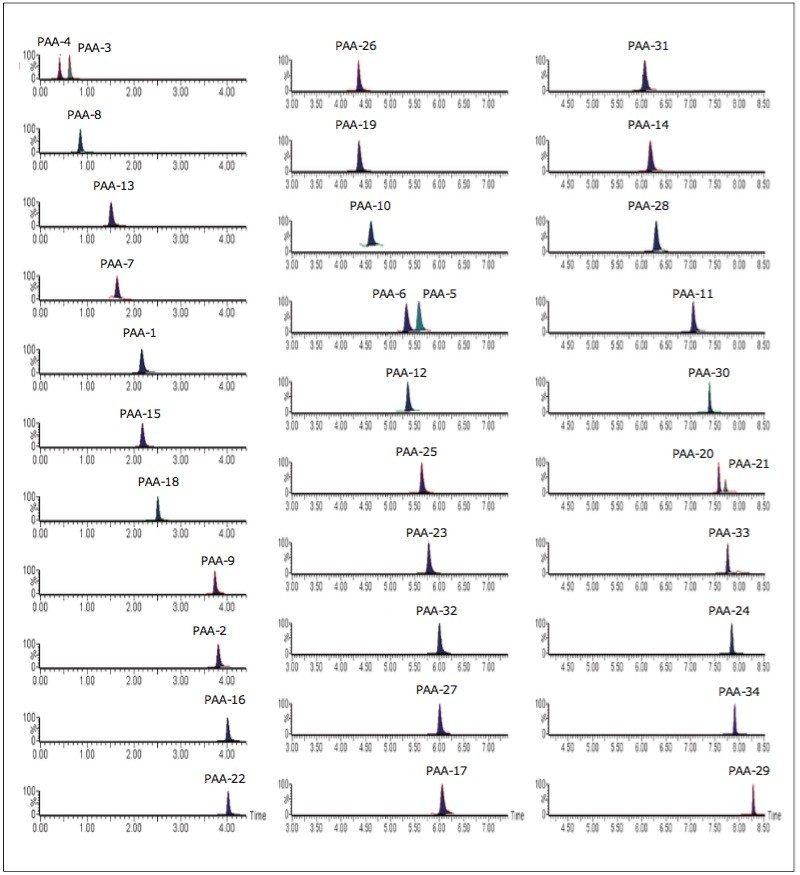
Mixed calibration standards were prepared and analyzed for all the PAAs considered. The TargetLynx Quantify results for aniline are shown in Figure 4, and the SIR chromatograms for each PAA are shown in Figure 5.
The SIR mass detection method detailed in Figure 1 was used after appropriate sample preparation to screen for PAAs in ink (containing PAAs) and paper (applied with ink containing PAAs).
Neat ink diluted 1:100 with 5% methanol/95% water was fortified at various levels with selected PAAs, and analyzed without any further cleanup or concentration steps. The results obtained are detailed in Table 3.
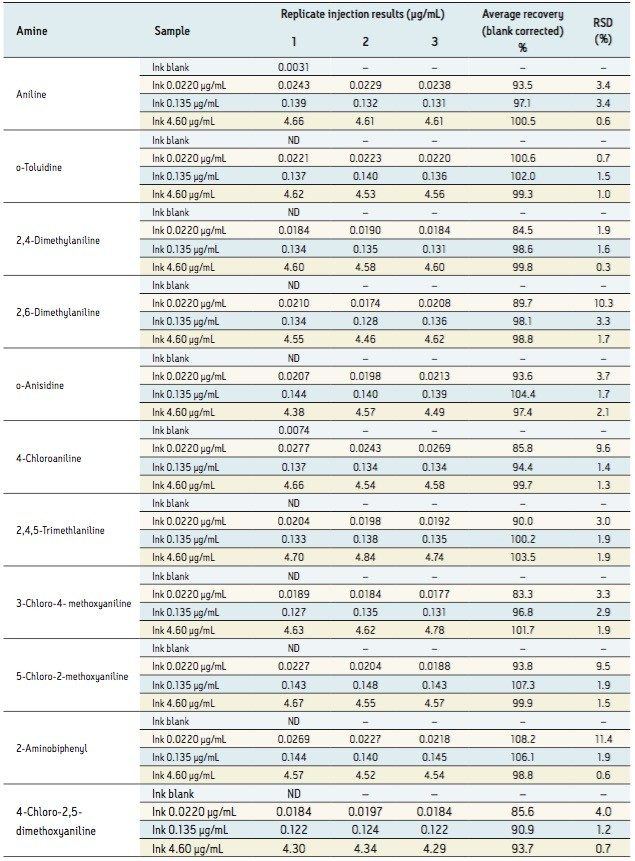
The efficient recoveries obtained (ranging between 83% to 108%) demonstrated that minimal signal enhancement/suppression was observed using ESI ionization for the analysis of PAAs within an ink matrix.
Within the food packaging industry great efforts are made to reduce food contamination in order to guarantee consumer safety and comply with regulations. The design of the packaging and the products used ideally afford minimal leaching and hence reduce potential contamination of the food product. Such packaging leachables have a large number of potential sources including PAAs from the ink used within the packaging.
In order to consider the EU regulations with regard to the release of total PAAs from food contact material, a cold water paper extraction based on the European standard (EN 646:1993) was used.
Three pieces of paper (10 cm x 10 cm) were taken, one kept as a blank and two applied with 100 μL ink previously fortified with selected PAAs. The paper was left to dry and then cut up and extracted in sealed containers with 100 mL of water and left for over 24 hours prior to analysis. The results obtained are detailed in Table 4.
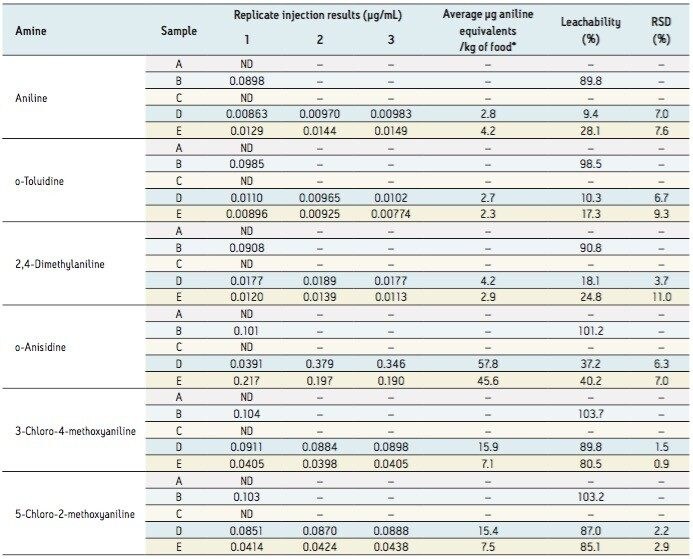
Table 4. Leachability results for paper previously applied with ink containing selected PAAs.
A = water blank, B = water containing 0.1 μg/mL PAAs, C = paper blank with no ink, D = paper applied with ink containing 10 μg PAAs, E = paper applied with ink containing 5 μg PAAs. *Calculated using a conventional surface area/volume conversion factor of 6 dm2/kg as established in the EU commission Directive 2007/19/EC.
Sample A results demonstrate that there were no residual PAAs in the water used or as background within the system. Sample B shows the efficacy of the extraction method used, as demonstrated by the high leachability recovery values observed (90% to 104%) when PAAs were added to the water with no paper present. The results most relevant to the food packaging industry were obtained for Samples C and D, which revealed the different extents to which the selected PAAs were being absorbed and not leached from paper.
720004151, December 2011