In order to ensure public health and safety, a reliable screening analysis is necessary to determine veterinary drug residue levels in meat and other edible tissue samples. The compounds of interest range from highly polar water-soluble compounds to very non-polar, fat-soluble compounds. There exist very effective extraction and cleanup procedures for individual compounds or compound classes, but these methods are not well suited for a multi-class, multi-residue screening analysis.
Optimized sample preparation and analysis protocols were developed for tandem LC/MS determination of a wide variety of veterinary drug residues in tissue samples. Three types of muscle tissue samples (pork, chicken, and salmon) were chosen to demonstrate the suitability of the methodology. Samples are treated with an acidified acetonitrile/water solvent to precipitate proteins and to extract the veterinary drugs of interest. Then, a simple SPE cleanup is performed using a Sep-Pak C18 cartridge or 96-well plate. After evaporation and reconstitution, the sample is analyzed using tandem LC-MS. Representative compounds were chosen from major classes of veterinary drugs including tetracyclines, fluoroquinolones, sulfonamides, macrolides, beta-lactams, NSAIDS, steroids, and beta-andrenergids.
|
System: |
ACQUITY UPLC system |
|
Column: |
ACQUITY UPLC CSH C18, |
|
1.7μm, 100 mm x 2.1 mm |
|
|
(i.d.) |
|
|
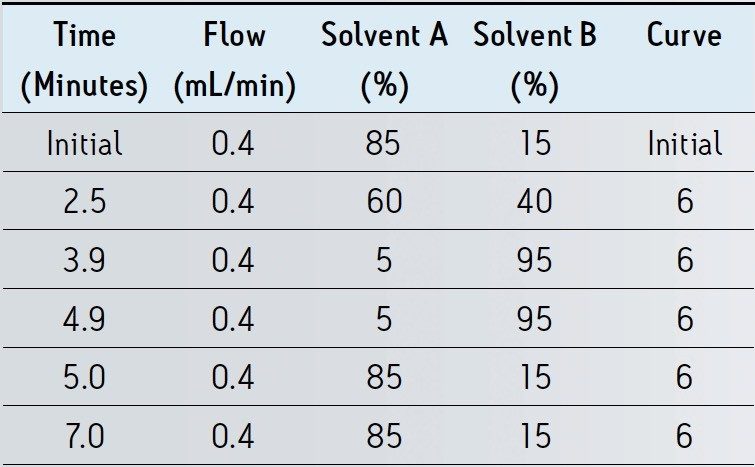
Mobile Phase: |
A: 0.1% formic in water |
|
B: 0.1% formic acid |
|
|
in acetonitrile |
|
|
Injection Volume: |
7 μL |
|
Injection Mode: |
Partial loop injection |
|
Column Temp.: |
30 °C |
|
Weak Wash: |
10:90 acetonitrile:water |
|
(600 μL) |
|
|
Strong Wash: |
50:30:40 |
|
water:acetonitrile:IPA |
|
|
(200 μL) |
|
|
Seal Wash: |
10:90 acetonitrile:water |
|
Detector: |
Waters Xevo TQ |
|
Ionization: |
Positive Electrospray (except negative for chloramphenicol) |
|
Source Temp.: |
150°C |
|
Desolvation Temp.: |
500°C |
|
Desolvation Gas Flow: |
1,000 L/hr |
|
Cone Gas Flow: |
30 L/hr |
|
Collision Gas Flow: |
0.15 mL/min |
|
Data Management: |
MassLynx v4.1 |
1. Initial Extraction/Precipitation
Place a 5 g sample of homogenized tissue into a 50 mL centrifuge tube. Add 10 mL 0.2% formic acid in 80:20 acetonitrile/water. Vortex for 30 seconds and place on mechanical shaker for 30 minutes. Centrifuge at 12000 rpm for 5 minutes.
The extraction/precipitation step gives good recovery of most compounds of interest but also extracts significant amounts of fat.
2. SPE Cleanup
Take 1 mL of the supernatant (from step 1) for SPE cleanup using a Sep-Pak C18 cartridge or plate (see SPE details in Figure 1).
This step removes fats and non-polar interferences.
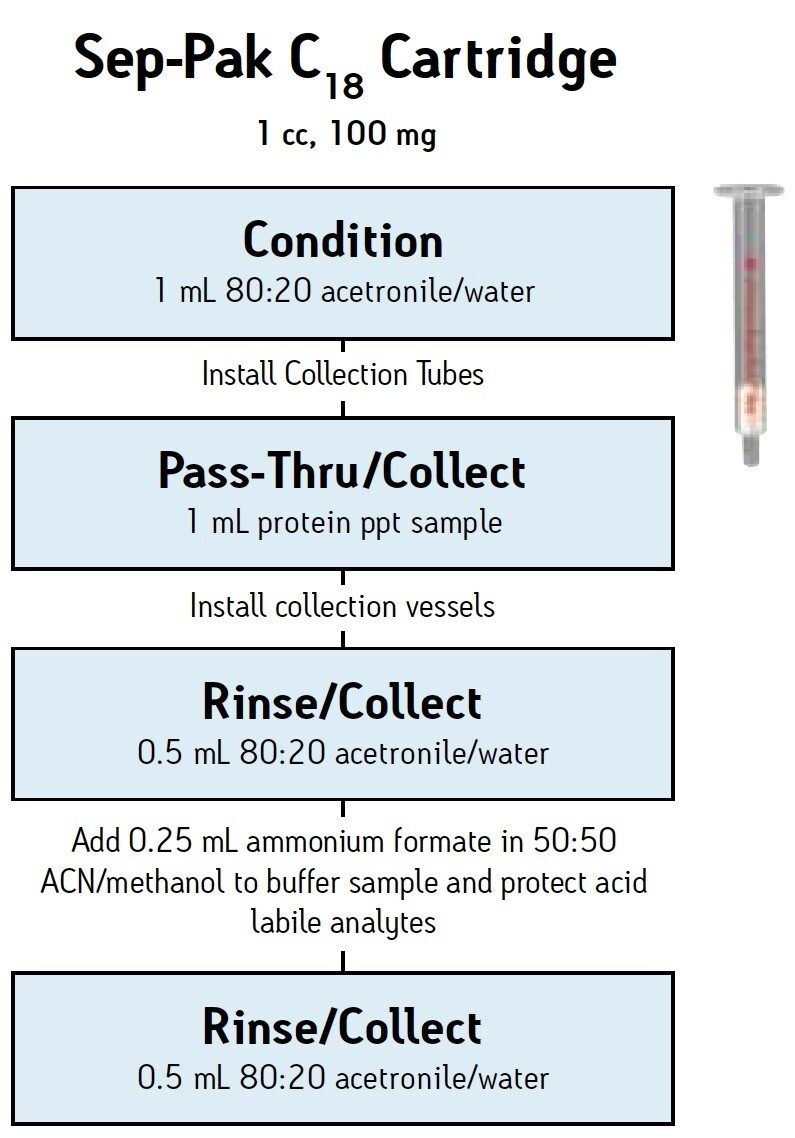
Table 1 summarizes the MRM transitions and instrument parameters used for this study. Also presented in Table 1 are typical matrix matched calibration data for each compound (calculated using the primary transition in pork matrix).
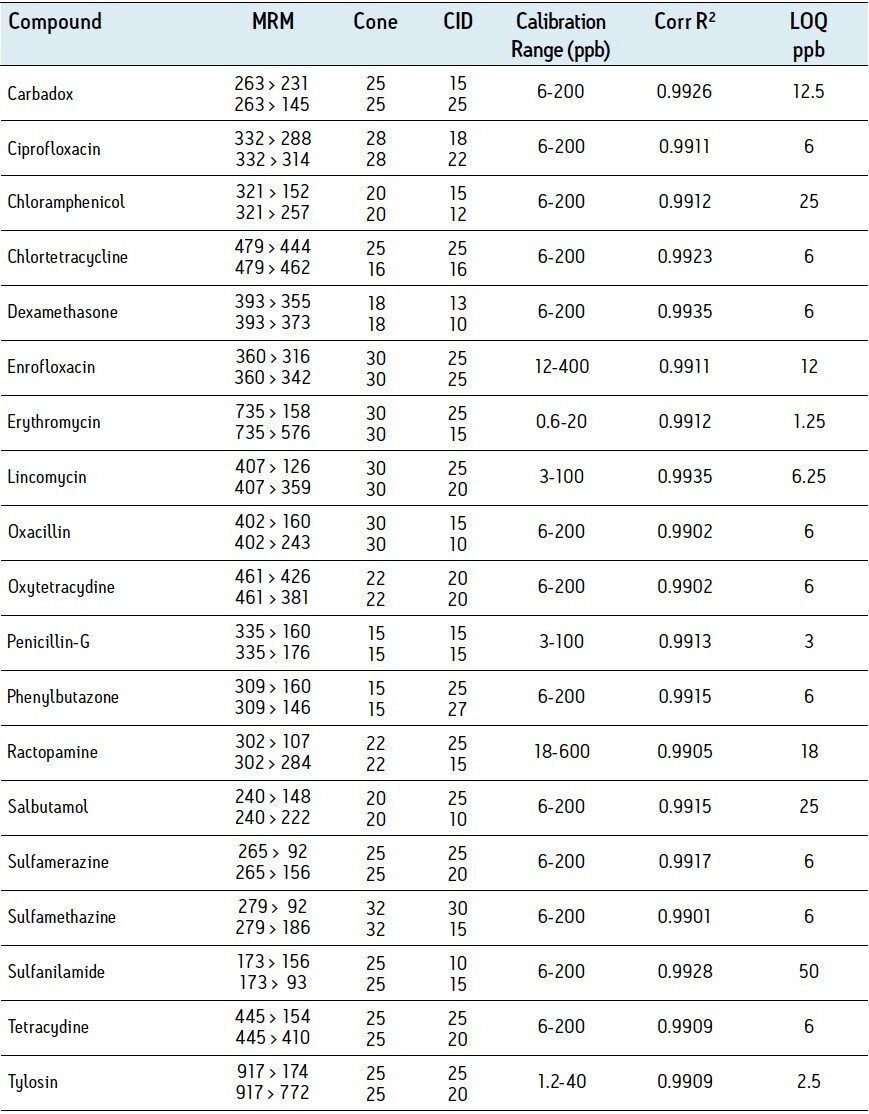
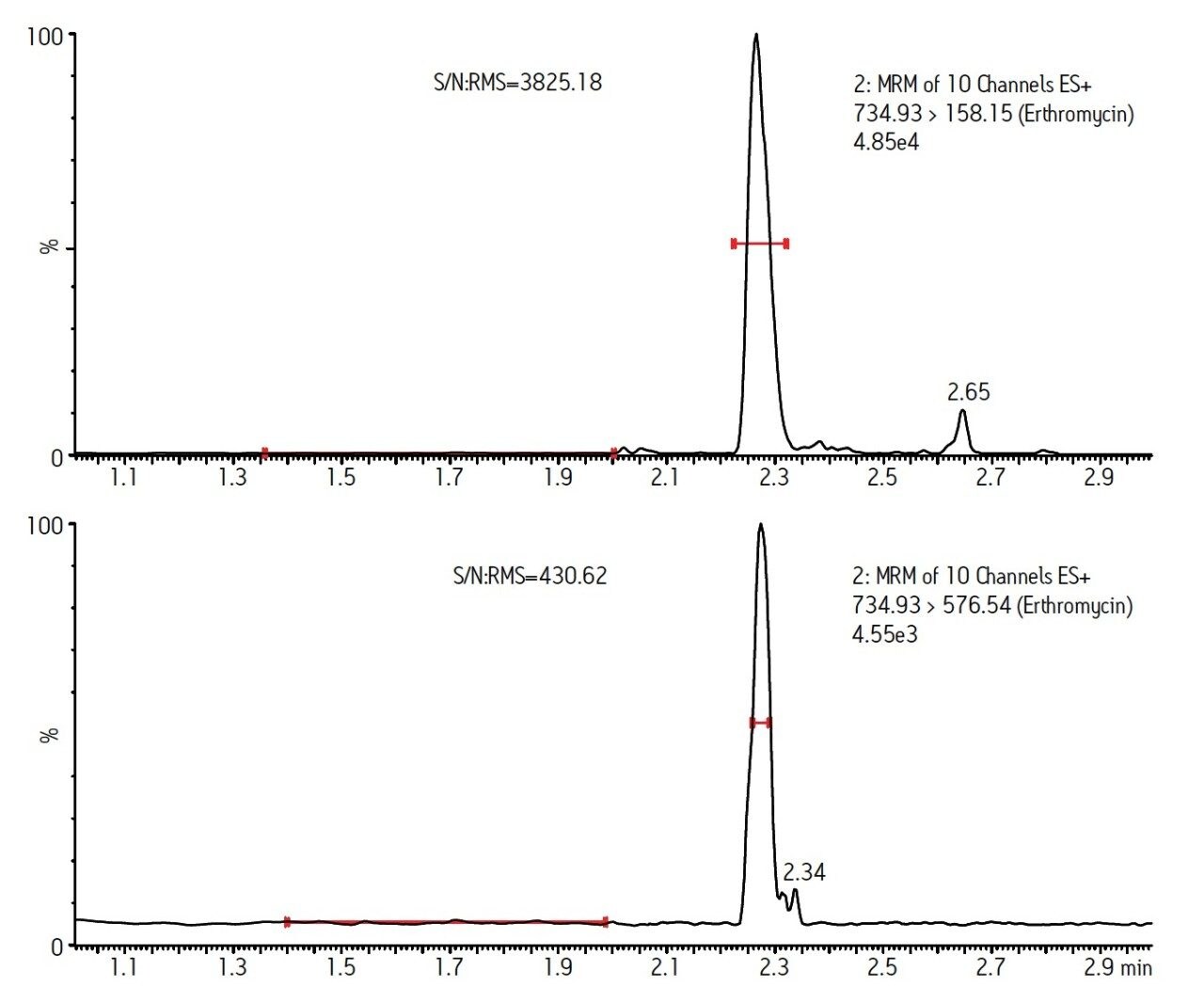
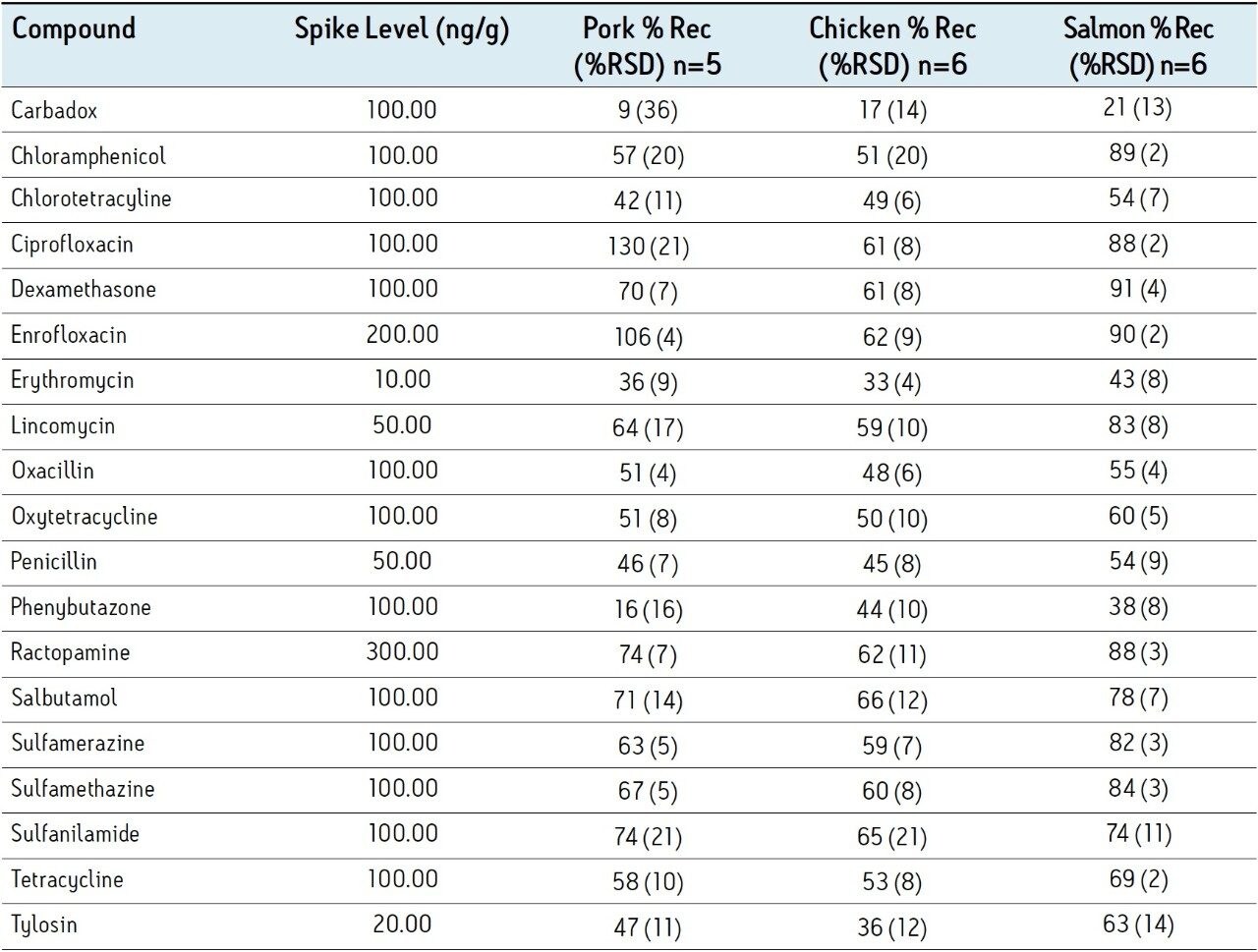
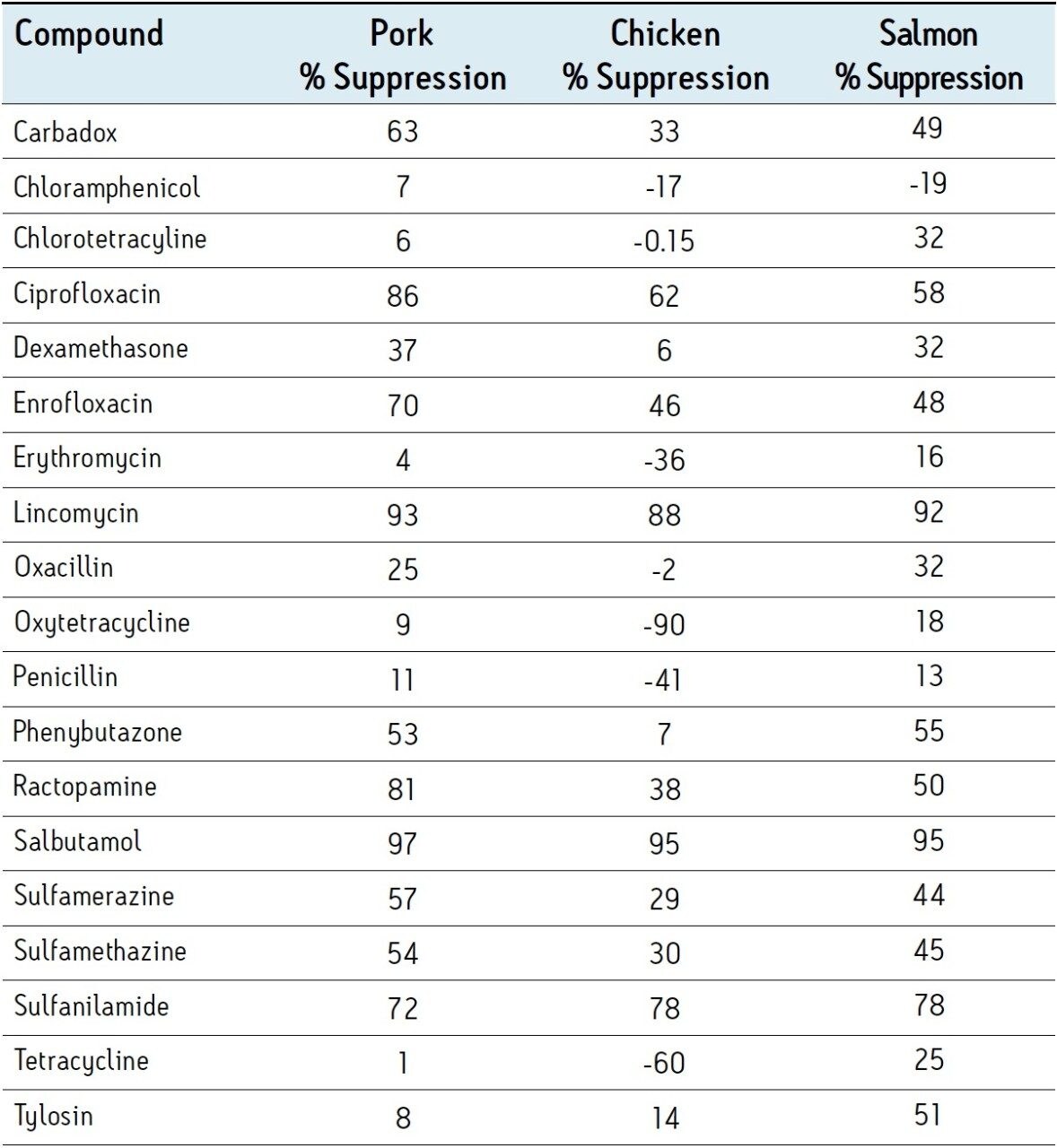
Figure 2 shows a typical LC-MS chromatogram obtained from analysis of a matrix matched standard of erythromycin at 10 ng/g. Performance of the other compounds was similar. Table 2 shows the recovery data obtained from replicate analysis of spiked tissue samples. Table 3 shows the observed matrix effects for the multi-residue tissue analysis.
The procedure utilized in this study was developed from methods presented by Lehotay et.al.1 and Martos et. al.2. The method used in reference 1 uses no acid in the tissue extraction solvent; under these conditions we observed recovery of tetracyclines below 5% and the RSD for recovery of fluoroquinolones was greater than 50%. The method used in reference 2 prescribes the acidification of the extract to 1% formic acid prior to centrifugation; under these conditions penicillin recovery was under 10% compared with 48% using our approach. Our extraction procedure is a compromise of the methods presented in reference 1 and 2. A similar acetonitrile/water based extraction is used but is acidified only to 0.2% with formic acid; more balanced recovery and minimized degradation of labile compounds was achieved.
Another approach was considered based on the method of Kauffman et. al.3 by which two separate extractions were performed. The first extraction, for the water soluble compounds, was accomplished using aqueous succinic buffer. The second, performed on the re-suspended pellet, was with acetonitrile. This approach requires that each fraction be worked up independently before ultimately combining fractions for a single injection. Performance was only marginally better than the chosen procedure but at a much greater cost of time and materials. The extraction, cleanup, and analysis protocols chosen for this study provide a good balance of preparative time, cost and method performance.
720004144, November 2011