This Application note demonstrates the use of the ASAP probe in conjunction with high resolution time-of-flight (ToF) MS can substantially reduce the time of analysis, producing qualitative results and identification of potential migrants with increased confidence.
The use of the ASAP probe can substantially reduce the time of analysis, producing qualitative results and identification of potential migrants with increased confidence when used in conjunction with high resolution MS detection techniques, such as time-of-flight (ToF) MS. The use of ToF-MS also allows full scan screening of the samples so potential migrants other than those specifically analyzed for may also be detected.
Most food and drink is packaged in some way. It is also highly likely that it comes into contact with other materials during harvesting, production, transport, storage, and cooking. A food contact material (FCM) is any material or article intended to be placed in contact with foodstuffs.1 Food packaging materials are the most notable example, but also included are cutlery, dishes and plates, containers, parts of food processing equipment, etc.
When food comes into contact with a FCM there is the potential for migration of any of the chemicals from the material into the foodstuff. Depending on the chemical substance(s) involved, this can compromise the safety and/or the quality of the food, and so most countries have legislation in place to keep any chemical migration within acceptable limits. In Europe the EU Framework Regulation (EC) No. 1935/20042 provides general requirements for FCMs. Article 3 states that they should not endanger human health, bring about an unacceptable change in composition, or deteriorate any organoleptic characteristics.
Further to this framework regulation is more specific legislation. One example is the migration of primary aromatic amines (PAAs) which are regulated through the Plastics Directive 2002/72/EC3, as amended, which states that:
Over the last couple of years there have been numerous notifications relating to the migration of PAAs from nylon kitchen utensils via the Rapid Alert System for Food and Feed4 (RASFF). As concerns to human health grow regarding these FCMs, quicker and easier methods need to be developed to screen for compounds in the current legislation. This application note will detail the analysis of nylon kitchen utensils for PAAs and will show how the latest advances in mass spectrometer probe design help to achieve this goal.
|
LC-MS system: |
ACQUITY UPLC with Xevo G2 QTof (used in Tof mode) |
|
Ionization mode: |
ASAP + |
|
Corona current: |
1.0 μA |
|
Sample cone: |
30 V |
|
Source temp.: |
120 °C |
|
Desolvation gas: |
Nitrogen, 800 L/Hr, 500 °C |
|
Cone gas: |
Nitrogen, 5 L/Hr |
|
Lock mass compound: |
Leucine enkephalin, m/z 556.2771 |
|
Flow rate: |
10 μL / min |
|
Capillary voltage: |
3 V |
|
Collision energy: |
6 eV |
The samples tested were two black nylon kitchen utensils, a typical example is shown in Figure 1.

Variables such as cone voltage, desolvation gas (nitrogen) temperature and corona pin current were optimized using solvent standards. Once the optimum settings were achieved the screening of the sample took a matter of minutes. The ASAP probe was used in the usual way; a new glass capillary was used for each sample removing sample carryover giving results that were more reliable by minimizing false positives.
The glass capillary was inserted into the source chamber at an elevated temperature for approximately one minute. This cleaned any contamination from the tip. The probe was then removed, cooled and the glass tip wiped backwards and forwards across the surface for 10 seconds. The mass spectrometer was set to an optimum desolvation gas temperature and the probe reinserted into the Xevo G2 QToF and the signal created recorded. This manual screening process was performed as quickly as 3 minutes per sample.
Keeping a check on the migration of all the starting substances that may be used to make FCMs is a massive undertaking. This involves the chemical analysis of either the material itself or testing for migration of chemicals into foods or into model foods that are called food simulants. For this mass spectrometric methods and especially gas chromatography with mass spectrometric detection (GC-MS) and liquid chromatography with mass spectrometric detection (LC-MS) are widely used.
The use of the ASAP probe can substantially reduce the time of analysis, producing qualitative results and identification of potential migrants with increased confidence when used in conjunction with high resolution MS detection techniques, such as time-of-flight (ToF) MS. The use of ToF-MS also allows full scan screening of the samples so potential migrants other than those specifically analyzed for may also be detected.
Two different sampling techniques were tested to see which would achieve the better results. The ASAP probe was wiped across the surface of the kitchen utensils and then inserted into the MS. A fine powder was also prepared from the sample using sandpaper and the probe rubbed in this powder before insertion in to the MS. The strongest signal was seen for the powder approach, and the results for the two samples are shown in Figure 2.
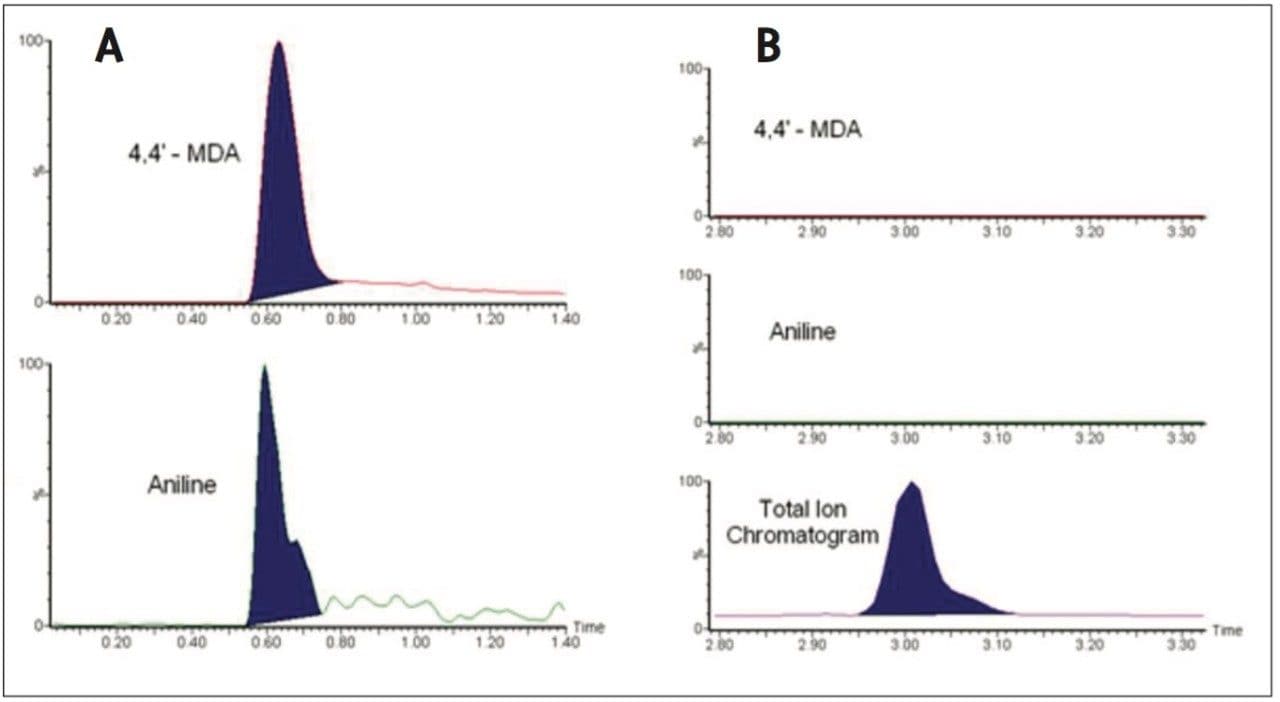
Sample A was found to contain levels of aniline and 4,4’-MDA ([M+H]+ adduct seen in both cases). PAAs were not detected in sample B. The total ion chromatogram gives the location of the peak on the trace, showing that the compounds are not present. These were the only compounds to give a positive result for these samples.
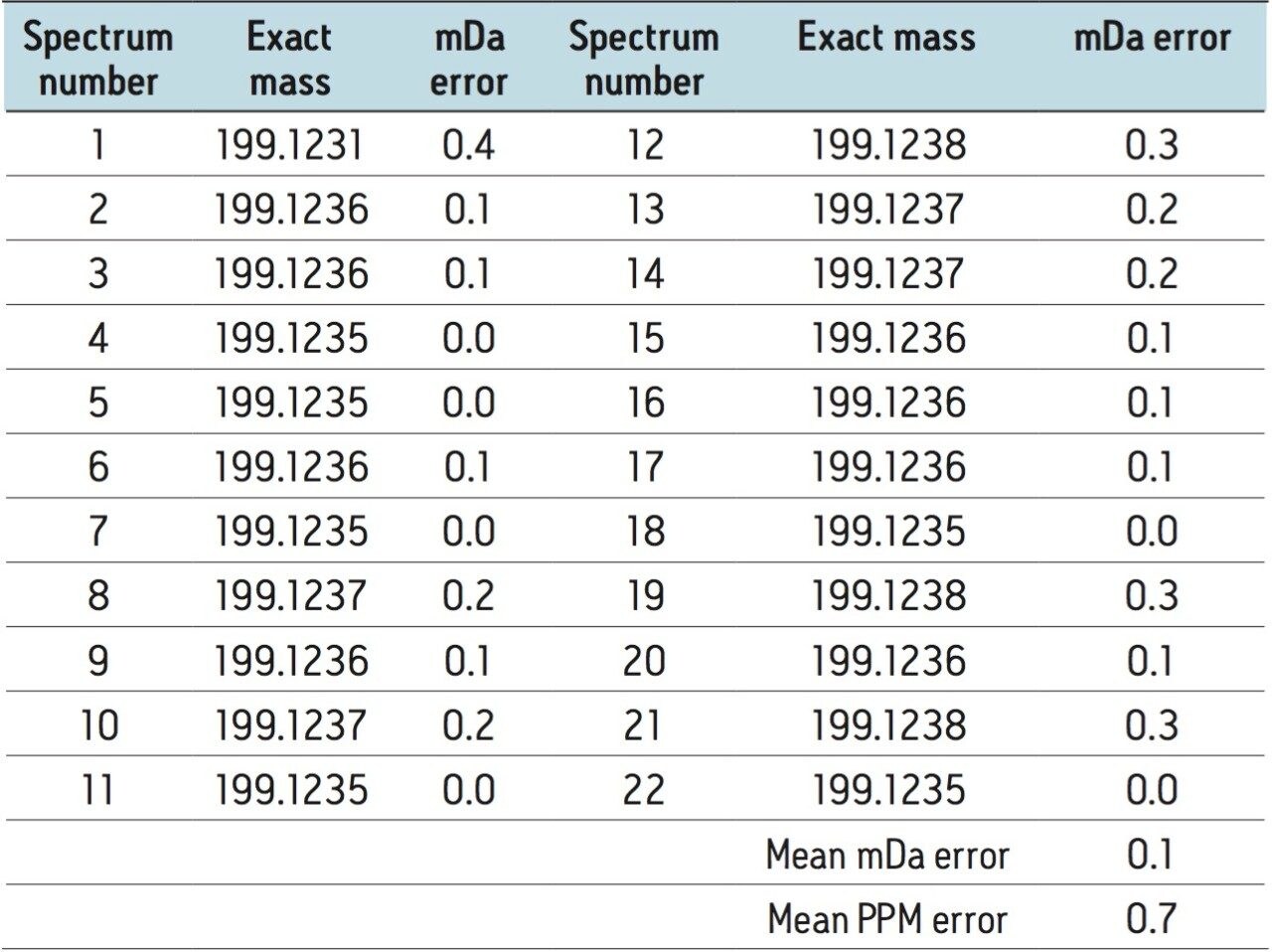
A high degree of confidence was achieved with the identification of these compounds. All of the spectra across the 4,4’-MDA peak were assessed with respect to mass accuracy of the system. Figure 3 shows the spectrum acquired at the apex of the peak (spectrum 11), the total mass accuracy across the peak is shown in Table 1.
Having identified sample A as a potential positive, it clearly merits being subjected to migration testing using food simulants to see if it complies or not with migration limits for the PAAs identified.

This data was acquired using a Xevo G2 QToF in ToF mode. Further analysis of the data after it has been acquired is possible. In this example, the aim of the experiment was to look for PAAs, but examination of the ToF data revealed other potential migrants that were identified. Post acquisition interrogation of this sort would not be possible if a quadrupole MS system was used for the analysis that only acquired the data in SIR or MRM modes.
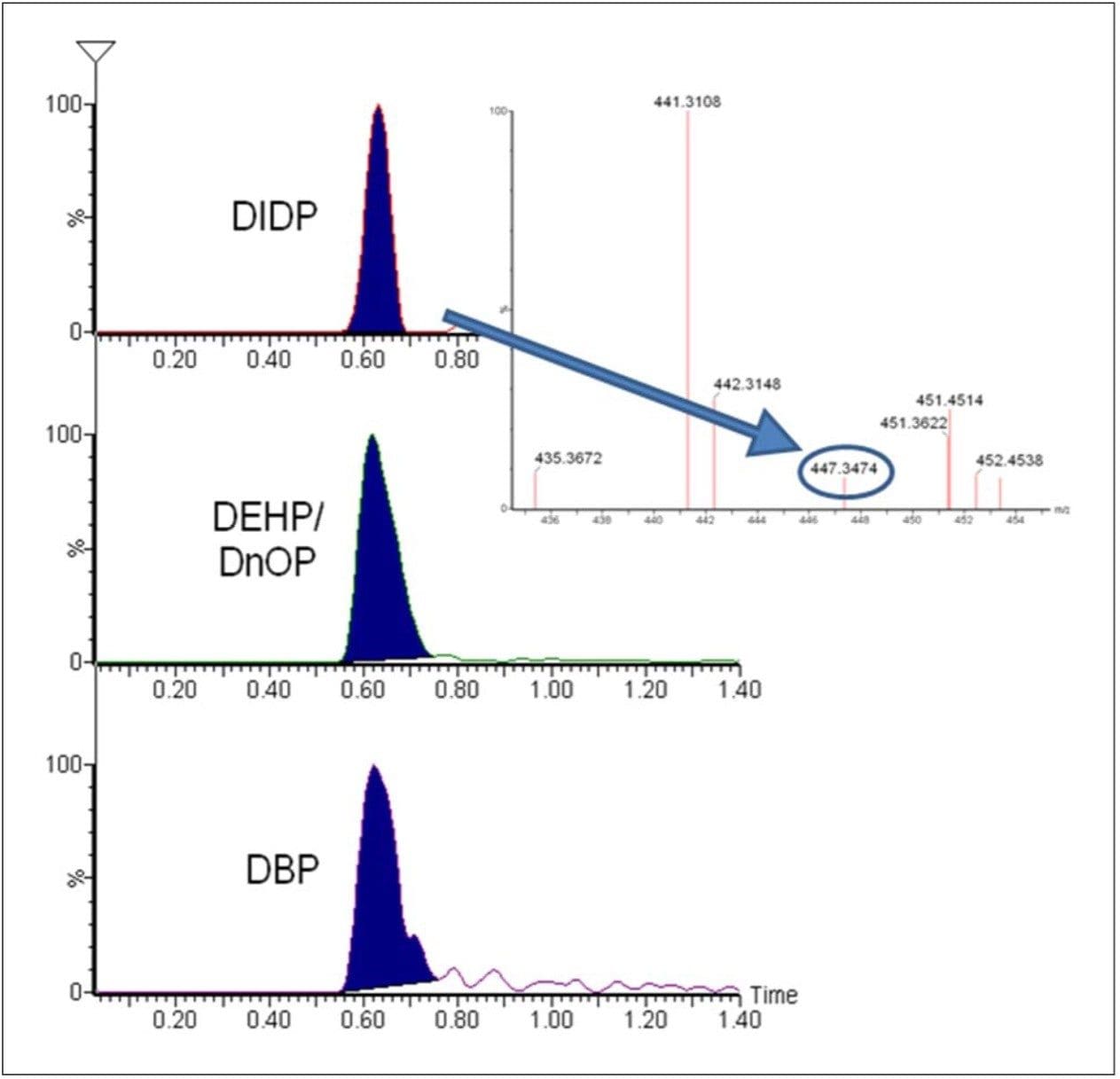
The presence of some common phthalates in sample A is shown in Figure 4. A chromatographic separation is needed to allow quantification of the isobaric DEHP and DnOP. As phthalates are ubiquitous in the environment the presence of phthalates may be due to contamination of the nylon sample. Further abrasion and testing would prove the origin.
720003829, January 2011