This application note describes the total mAb quantification of the antibody-drug conjugate, ado-trastuzumab emtansine, and the mAb, trastuzumab from rat plasma using the ProteinWorks eXpress Direct Digest Kit and Protocol.
The ProteinWorks eXpress Direct Digest Kit was successfully used for sample preparation prior to LC-MS quantification of trastuzumab and T-DM1 from a typical set of standard curve and QC samples in plasma. The universal, kit-based approach to sample prep allows novice users to achieve high LC-MS sensitivity with a simple step-wise protocol and standardized, premeasured reagents, ensuring both the analytical sensitivity and reproducibility required in discovery studies to make time sensitive and critical project decisions.
Simple, standardized approach for accurate and reproducible quantification of ADC and mAb therapeutics; broadly applicable optimized digest kit eliminates method development.
Monoclonal antibodies (mAbs), as well as antibody-drug conjugates (ADCs) represent a growing class of therapeutics due to their target specificity, lower toxicity and higher potency. With the increasing interest in mAb and ADC therapeutics, the desire for LC-MS bioanalytical quantification in support of drug development is also increasing. Historically, mAbs and ADCs have been quantified using ligand binding assays (LBAs), such as the gold-standard ELISA. While these immuno-based methods are sensitive and simple to execute, poor reagent reproducibility, lack of standardization, cross-reactivity, limited linear dynamic range, and other short-comings have led the drive to convert to LC-MS. In contrast, MS based methodologies offer many advantages over traditional LBAs, such as: multiplexing, broad dynamic range, superior selectivity, and shorter method development times. However, for LC-MS protein quantification challenges still exist. There is no single standardized workflow and the various workflow options can be complex and laborious, making it difficult for the novice bioanalytical scientist to achieve success. Additionally, due to their complex and heterogeneous nature, ADCs often require multiple bioanalytical assays to determine efficacy, toxicity, and PK/PD response during drug development stages. The bottom up approach, using enzymatic digestion of the ADC/mAb, followed by LC-MS/MS analysis is becoming routine for ADC and mAb quantification. Of the many experiments required to characterize and quantify ADC’s, total antibody measurements are important. This application note describes the total mAb quantification of the ADC, ado-trastuzumab emtansine, and the mAb, trastuzumab, from rat plasma using the ProteinWorks eXpress Direct Digest Kit and Protocol.
To prepare standards and quality control samples (QC), trastuzumab or T-DM1 was spiked into rat plasma at various concentrations (0.1–500 μg/mL). An intact murine monoclonal antibody standard (p/n 186006552) was used as a generic internal standard. Plasma samples (35 μL) were then prepared for LC-MS analysis using the ProteinWorks eXpress Direct Digest Kit and a 5-step digestion protocol which included reduction and alkylation.
|
LC system: |
ACQUITY UPLC |
|
Detection: |
Waters Xevo TQ-S Mass Spectrometer, ESI+ |
|
Column: |
ACQUITY UPLC Peptide BEH C18, 300A 1.7 μm, 2.1 x 150 mm Column |
|
Temp.: |
55 °C |
|
Sample temp.: |
10 °C |
|
Injection vol.: |
10 μL |
|
Mobile phases: |
A: 0.1% formic acid in water |
|
B: 0.1% formic acid in acetonitrile |
|
|
Data management: |
MassLynx (v4.1) |
|
Flow rate (mL/min) |
Time (min) |
%A |
%B |
Curve |
|---|---|---|---|---|
|
0.3 |
0.0 |
100 |
0 |
6 |
|
0.3 |
1.0 |
100 |
0 |
6 |
|
0.3 |
16.0 |
50 |
50 |
6 |
|
0.3 |
16.5 |
10 |
90 |
6 |
|
0.3 |
17.5 |
10 |
90 |
6 |
|
0.3 |
18.0 |
100 |
0 |
6 |
|
0.3 |
20.0 |
100 |
0 |
6 |
|
Capillary (kV): |
3 |
|
Cone (V): |
30 |
|
Source offset (V): |
50 |
|
Source temp. (°C): |
150 |
|
Desolvation temp. (°C): |
600 |
|
Cone gas flow: (L/Hr): |
150 |
|
Desolvation gas flow: (L/Hr): |
1000 |
|
Collision gas flow (mL/Min): |
0.15 |
|
Nebuliser gas flow (Bar): |
7 |
Trastuzumab is a humanized anti-HER2 monoclonal antibody that was approved by the FDA in 1998. With EU patent expiry in July 2014, and impending US patent expiry in 2019, the focus on this drug, as well as next and new generation drugs, such as ADCs, has steadily increased. Ado-trastuzumab emtansine (T-DM1) is an FDA approved ADC, marketed under the brand name Kadcyla, and used as treatment for patients with advanced breast cancer.1-3 ADCs, like T-DM1, are composed of cytotoxic small molecule drug (payload) covalently bound to an antibody by a linker. Due to their complex and heterogeneous nature, ADCs require multiple bioanalytical assays to quantify both conjugated and unconjugated forms of the ADC, total mAb, cytotoxic payload, and various other catabolites/metabolites. LC-MS quantification of ADCs and mAbs typically employs enzymatic digestion (most commonly trypsin), followed by quantification of one or multiple representative tryptic peptides using multiple reaction monitoring (MRM).
Using the ProteinWorks eXpress Direct Digest Kit and protocol, a direct digest of plasma (35 μL) containing either T-DM1 or trastuzumab was performed. LC-MS/MS quantification of signature peptides was performed using a Xevo TQ-S triple quadrupole MS. Chromatographic separation was achieved using an ACQUITY UPLC System with an ACQUITY UPLC Peptide BEH C18, 300A, 1.7 μm, 2.1 x 150 mm Column. Three signature tryptic peptides were used for quantification: IYPTNGYTR, FTISADTSK, and GPSVFPLAPSSK. MS conditions are summarized in Table 1.
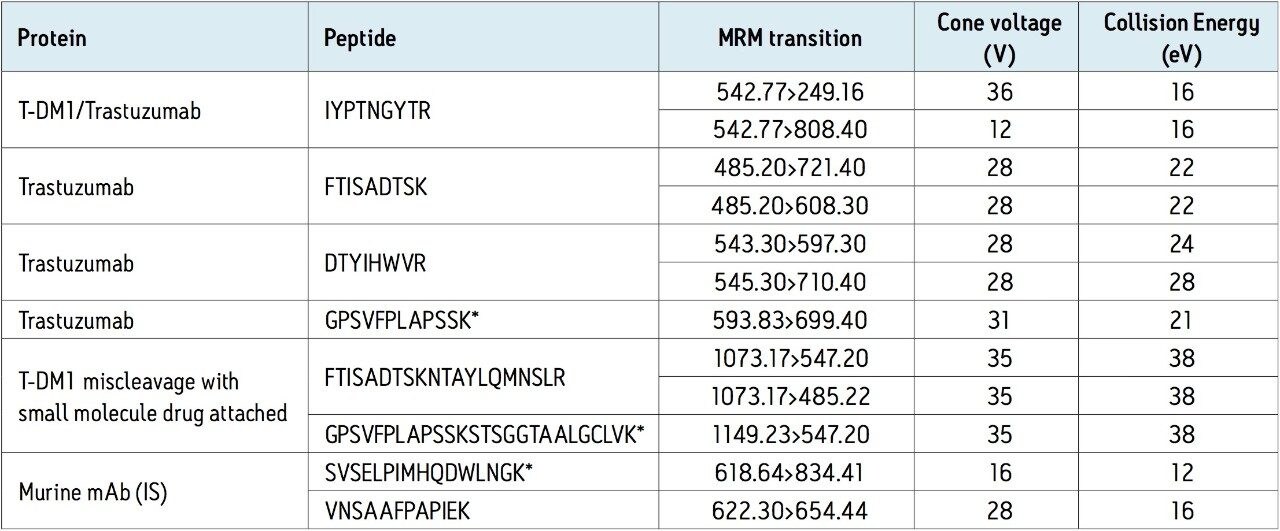
From an analytical perspective, tryptic digestion and choice of signature peptide poses a challenge for quantification of T-DM1, since it is a lysine-conjugated ADC. Trypsin cleaves peptides on the C-terminal side of lysine amino acid residues and if a lysine residue is occupied with the cytotoxic drug, cleavage will not occur (“miscleavage”). Thus, if one were to choose a lysine containing peptide to quantify T-DM1, there is potential for miscleavage on the lysine residue when it is conjugated with the small molecule drug. Because the signature peptide IYPTNGYTR lacks a lysine residue, one can confidently and accurately use it to quantify both T-DM1 and trastuzumab. For this same reason one would need to be cautious of using the two lysine containing peptides, FTISADTSK and GPSVFPLAPSSK for accurate quantification of T-DM1. Both of these peptides have some degree of small molecule drug occupancy and thus, due to potential miscleavage of the lysine residue may result in lower calculated concentrations than a non-lysine containing peptide.
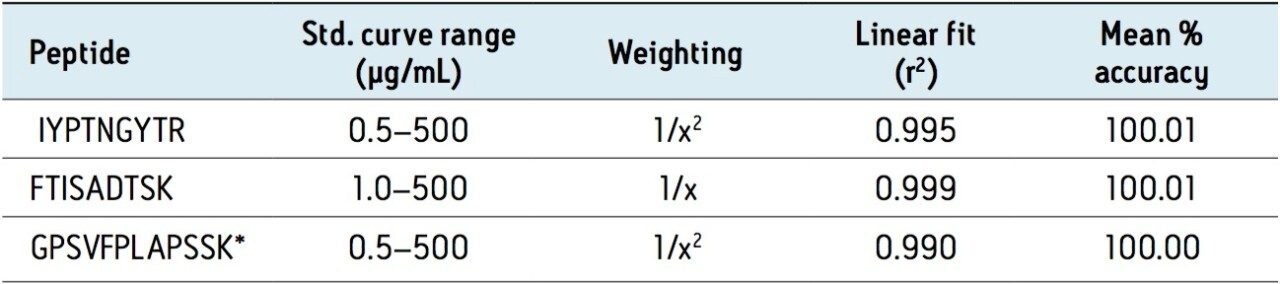
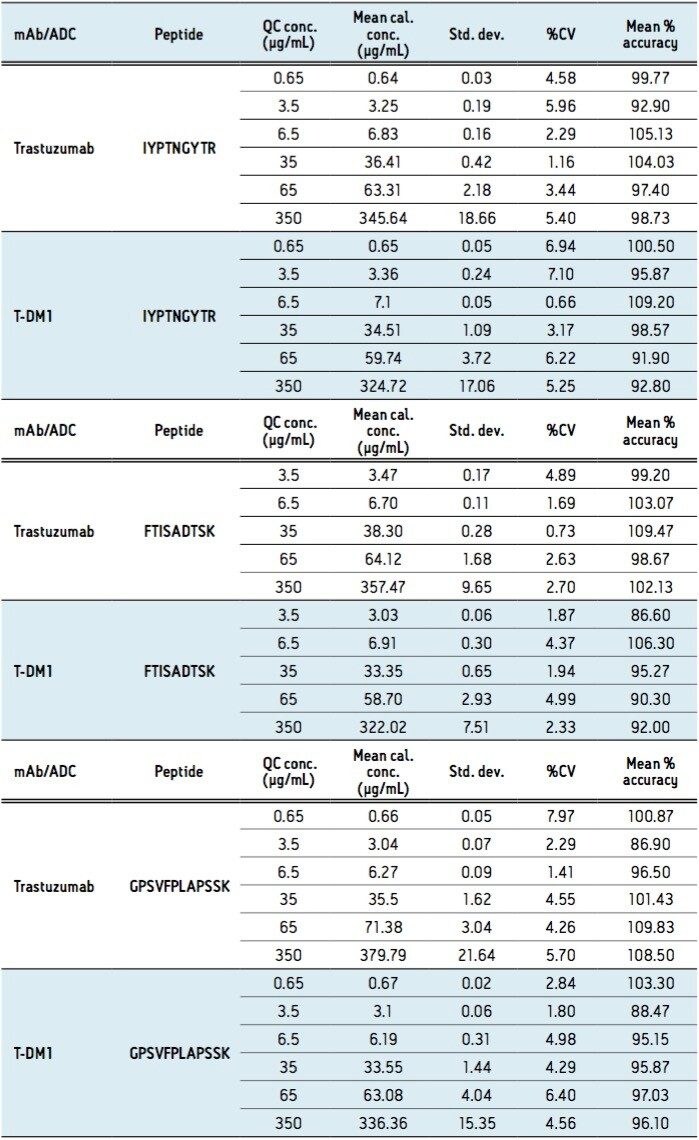
For this application, sensitivity, linearity, accuracy and precision data met typical method validation requirements.4 Standard curves were linear over 3.5 orders of magnitude with the average accuracies of 100% for the standard curve points. For the IYPTNGYTR, FTISADTSK, and GPSVFPLAPSSK tryptic peptides, quantification limits between 0.5–1.0 μg/mL were achieved. Summary statistics from standard curves for trastuzumab are shown in Table 2. In addition, the accuracy and precision for trastuzumab and T-DM1 QC samples, quantified using the trastuzumab standard curve, were excellent with % CVs <8. This is summarized in Table 3.
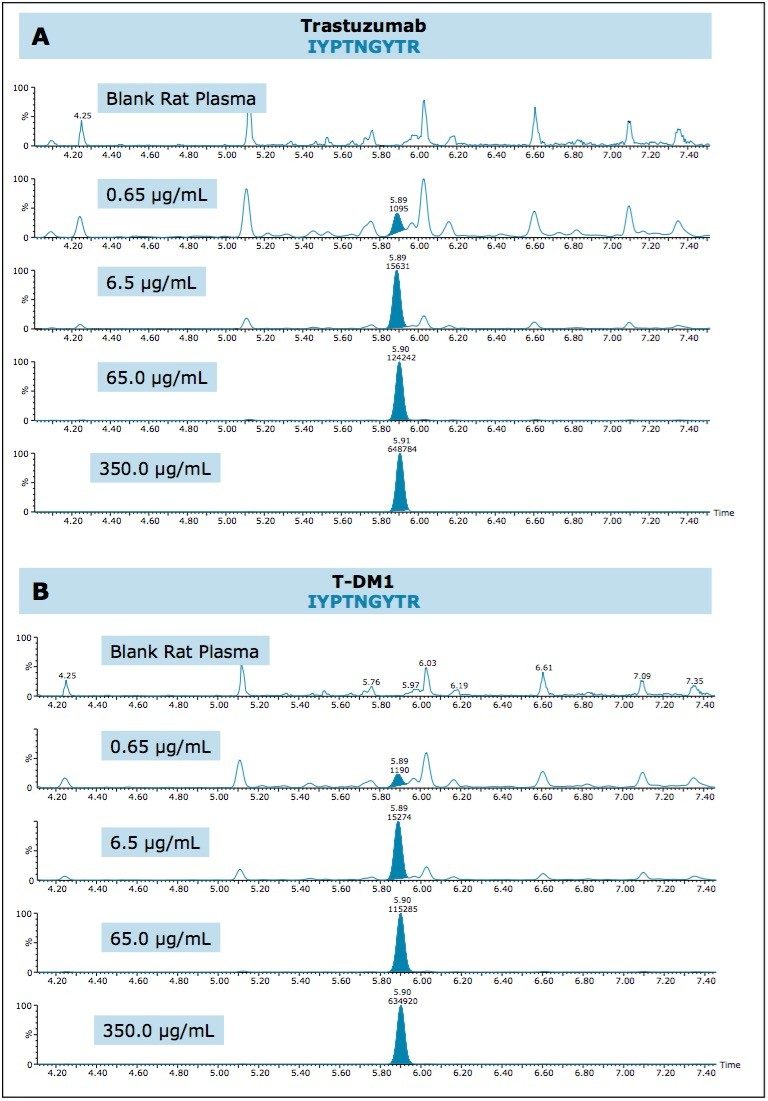
QC chromatographic performance and demonstration of sensitive quantification for all three signature peptides is highlighted in Figures 1–3, Panels A (trastuzumab) and B (T-DM1), respectively.
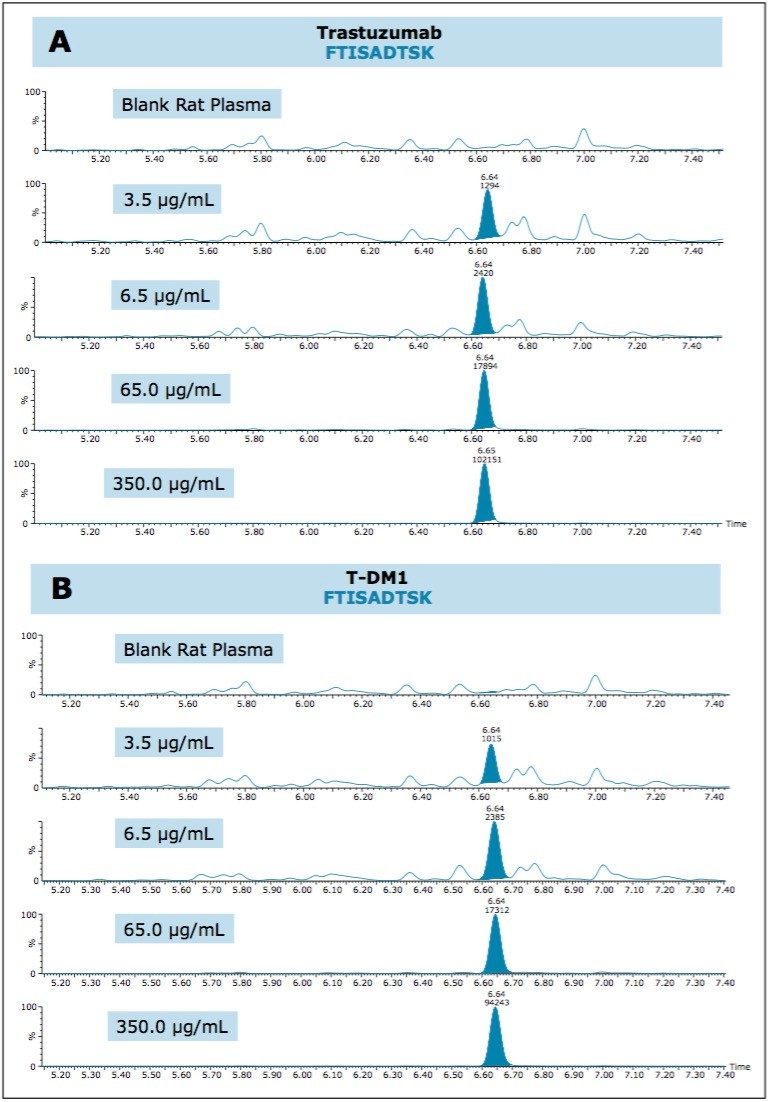
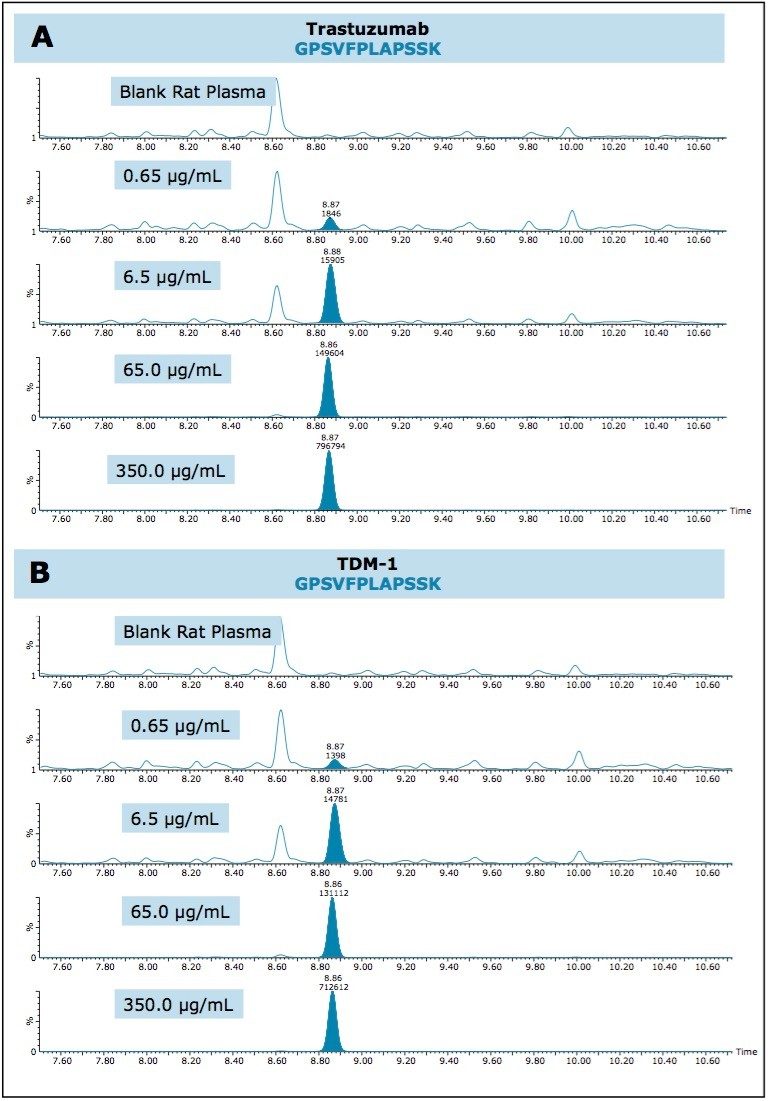
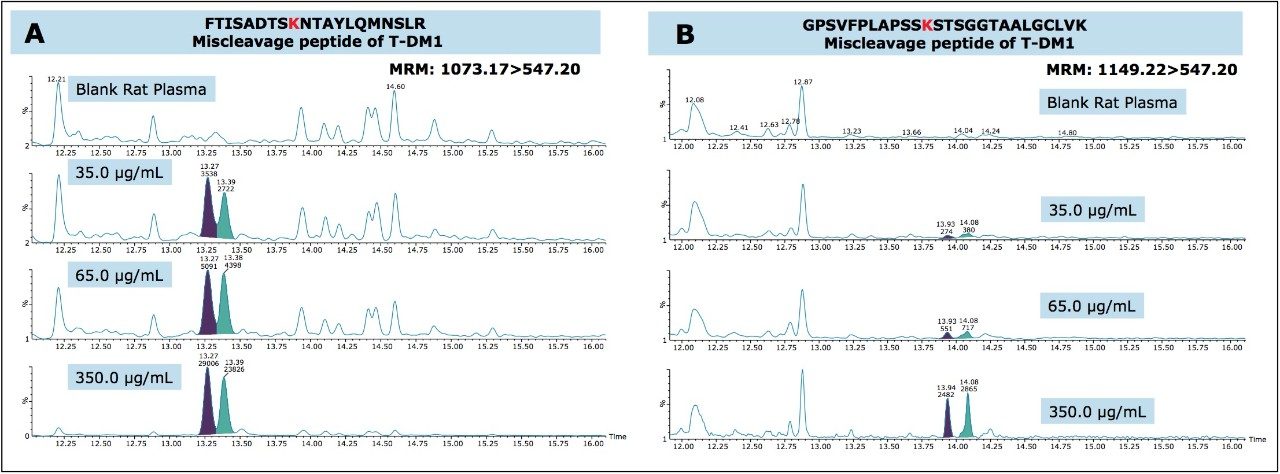
Due to the hydrophobic nature of the cytotoxic drug molecule attached to the antibody and differences in stereo chemical configurations, conjugated TDM-1 peptides generally will elute later in a chromatographic run as diastereomeric pairs. Additionally, TDM-1 peptides, by collision induced disassociation (CID), produce a common fragment (547.2 m/z). This fragment corresponds to part of the drug molecule broken down by the CID process.
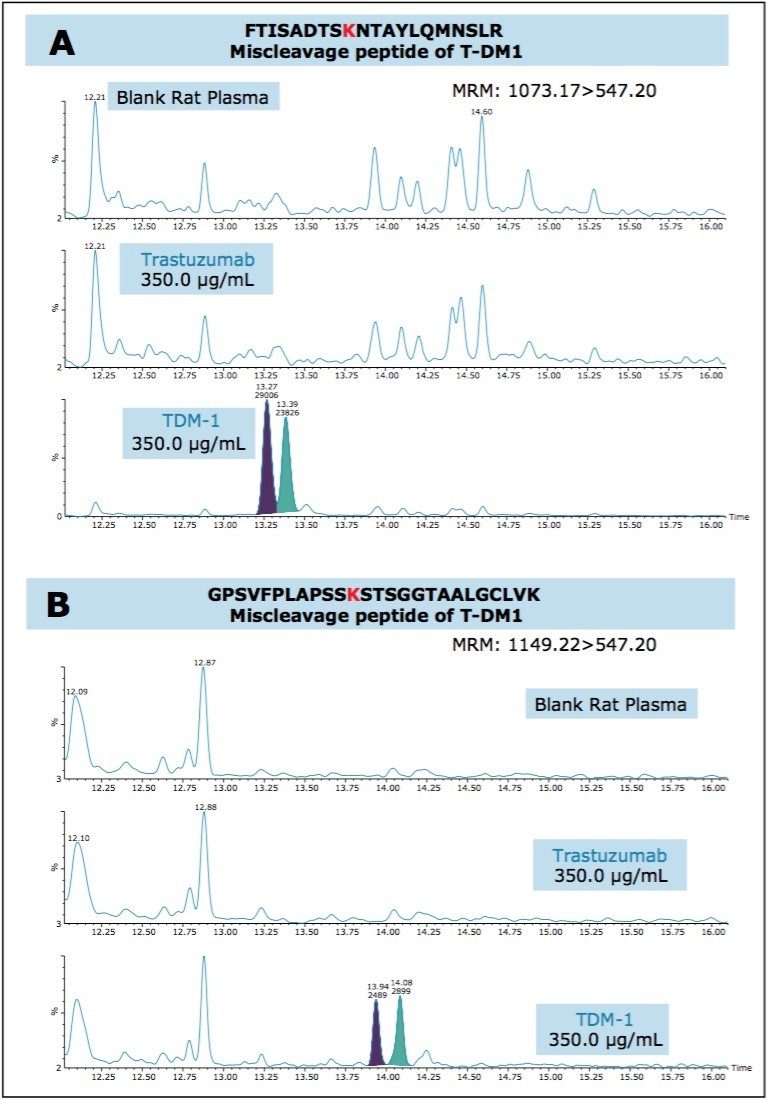
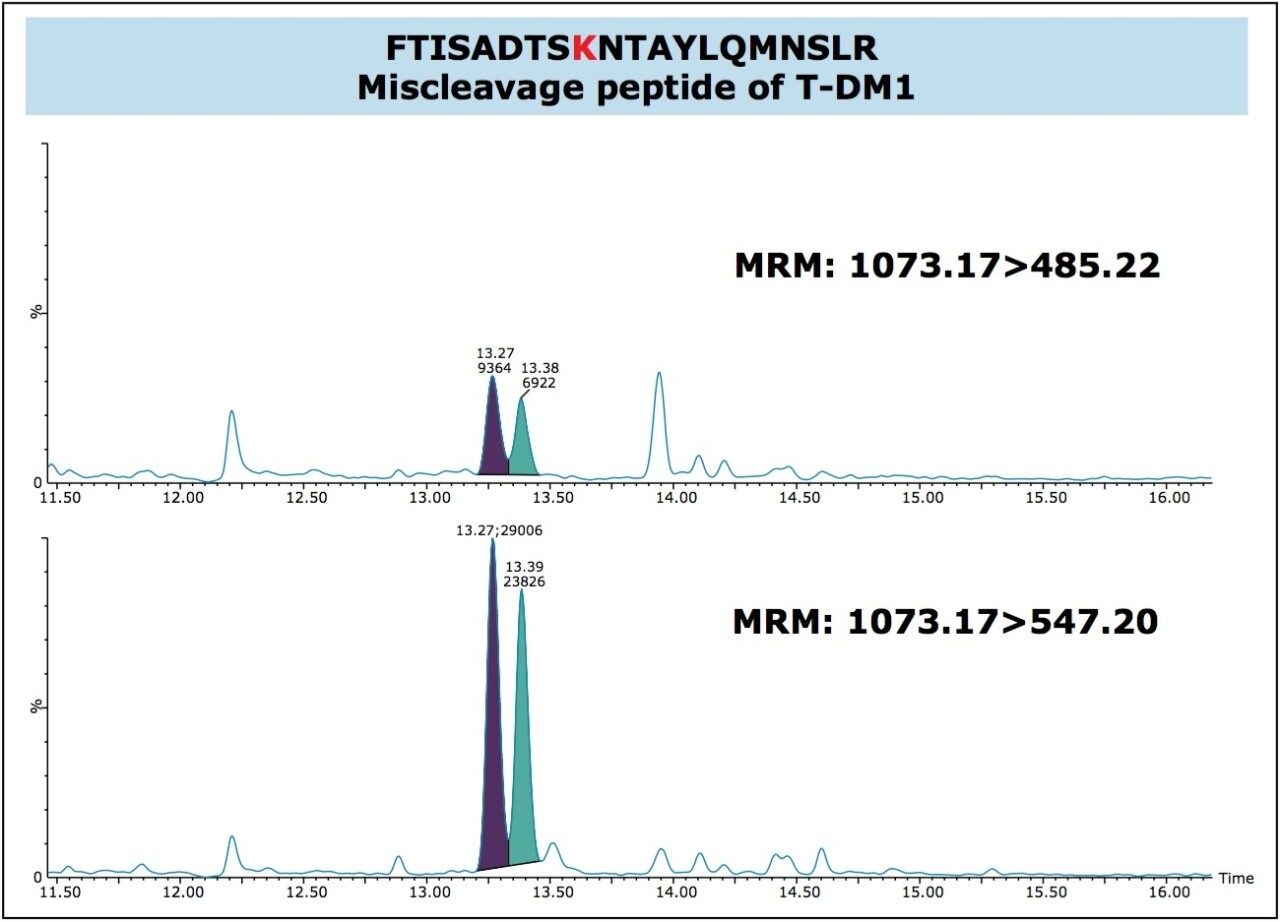
In this application, we were successfully able to detect two conjugated, “miscleavage” peptides of TDM-1 (FTISADTSKNTAYLQMNSLR and GPSVFPLAPSSKSTSGGTAALGCLVK). These conjugate peptides contained a common fragment 547.2 m/z from the conjugated payload and eluted later in the chromatographic run, as pairs (isomers from the conjugation). Figure 4, panels A and B illustrate the presence of these conjugated peptides in TDM-1 plasma samples (350 μg/mL), as compared to Trastuzumab (350 μg/mL), and blank rat plasma. Presence of the FTISADTSKNTAYLQMNSLR conjugated peptide was confirmed by multiple MRM transitions, and is shown in Figure 5. Additionally, both of these conjugated TDM-1 peptides increased with increasing concentration of T-DM1. This is highlighted in Figure 6, panels A and B.
The ProteinWorks eXpress Direct Digest Kit was successfully used to quantify trastuzumab and the ADC, T-DM1, from a typical set of standard curve and QC samples in plasma. Through direct digestion of 35 μL of plasma, quantification limits of 0.5–1.0 μg/mL were achieved, while maintaining excellent linearity, precision and accuracy. The universal, kit-based approach allows novice users to achieve high sensitivity with a simple step-wise protocol and standardized, premeasured reagents, ensuring both the sensitivity and reproducibility required in discovery studies to make time sensitive and critical project decisions.
720005619, April 2016