In this application note, we explore the use of ion mobility with high resolution mass spectrometry as an important tool to gain a greater understanding of ion ratio variations. This technique offers some unique advantages to profiling complex matrices.
Current trends indicate that more than 500 pesticides are routinely used under strict regulation on a global basis. With increasing global trade there is a requirement for multi-analyte screening strategies that are capable of efficiently detecting residue violations to protect consumer safety. Different countries have respective regulations concerning licensing and Maximum Residue Limits (MRLs). The benefits of full spectra acquisition and the specificity of accurate mass measurements are well documented. These are used in combination with time tolerances, isotope fits, fragment ions/ratios, and response thresholds to reduce false positive/negative identifications in screening assays. Should a positive screening violation observation take place, confirmation is typically performed using the selective approach of MRM analysis on tandem quadrupole MS platforms.
Criteria used for confident compound identification include the determination of the acceptable product ion ratio tolerances and relative intensities of the detected ions. This is expressed as a percentage of the intensity of the most intense (abundant) ion or product ion, which should correspond to those of the calibration standard at comparable concentrations and measured under the same conditions. It is well known that ion ratio performance can vary with instrumentation parameters, as well as be affected by sample concentration and matrix. The SANCO/12571/2013 guidance document describes the method validation and analytical quality control requirements to support the validity of data used for checking compliance with MRLs, enforcement actions, or assessment of consumer exposure to pesticides in the EU.1
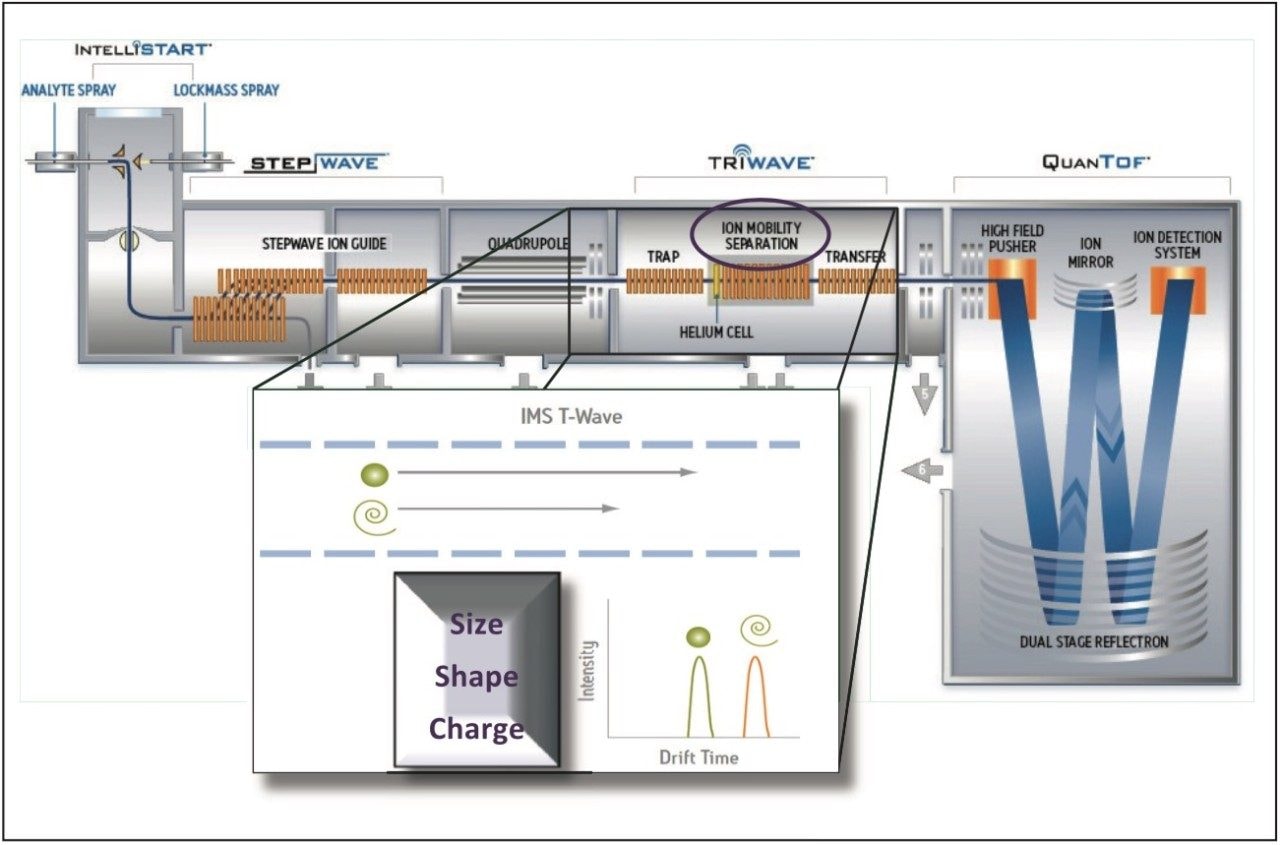
In this application note, we explore the use of ion mobility with high resolution mass spectrometry as an important tool to gain a greater understanding of ion ratio variations. This technique offers some unique advantages to profiling complex matrices. Ion mobility spectrometry (IMS) is a rapid, orthogonal, gas phase separation technique that allows another dimension of separation to be obtained within an UltraPerformance Liquid Chromatography (UPLC) timeframe. All analyte/matrix compounds can be differentiated based on size, shape, and charge. In addition, using the fastest UPLC compatible ion mobility duty cycle (10.8 ms), both precursor ion and fragment ion information can be acquired consecutively in a single injection.
|
System: |
ACQUITY UPLC I-Class |
|
Column: |
ACQUITY UPLC BEH C18 2.1 x 100 mm, 1.7 μm |
|
Column temp.: |
45 °C |
|
Flow rate: |
0.45 mL/min |
|
Mobile phase A: |
Water (0.1% formic acid) |
|
Mobile phase B: |
Acetonitrile (0.1% formic acid) |
|
Injection volume: |
5 μL |
|
Time (min) |
Flow rate (mL/min) |
%A |
%B |
|---|---|---|---|
|
0.00 |
0.45 |
98.0 |
2.0 |
|
0.25 |
0.45 |
98.0 |
2.0 |
|
12.25 |
0.45 |
1.0 |
99.0 |
|
13.00 |
0.45 |
1.0 |
99.0 |
|
13.01 |
0.45 |
98.0 |
2.0 |
|
13.00 |
0.45 |
98.0 |
2.0 |
|
17.00 |
0.45 |
98.0 |
2.0 |
|
System: |
SYNAPT G2-S |
|
Ionization mode: |
ESI+ |
|
Desolvation temp.: |
550 °C |
|
Mass range: |
50 to 1200 Da |
|
Acquisition rate: |
5 spectra/sec |
|
Capillary voltage: |
1 kV |
|
Cone voltage: |
20 V |
|
Drift gas: |
N2 |
|
Collision energy ramp: |
10 to 45 eV |
|
IMS wave velocity range: |
650 m/s |
|
IMS wave height: |
40 V |
|
IMS gas flow: |
90 mL/min |
|
IMS duty cycle: |
10.8 ms |
|
Lock mass: |
m/z 556.2766 (Leucine enkephalin) |
Empirically isobaric pesticide protomers have been identified and characterized using ion mobility. It is now possible to separate protomers (ions differentiated only by their protonation site) to determine their respective collision cross section (CCS) and individual protomer fragmentation dissociation pathways. This provides a unique view of the product ion formation information – by facilitating the selection of product ions will result in improved product ion ratio reproducibility. A schematic representation of the Waters SYNAPT G2-S Platform and an illustration of the mechanism of ion mobility are shown in Figure 1.
10 g of homogenized sample was extracted with 60 mL of 20-mM ammonium acetate in methanol using an Ultra-Turrax device. The crude extract was filtered and diluted up to 100 mL with 5 mM-ammonium acetate in water before injection.
An organic mandarin sample was used to produce a matrix matched calibration curve and a previous European ring-test FV-13 sample was analyzed (European Commission proficiency tests for pesticide residues in fruits and vegetables. FV-13 Mandarin Homogenate, 2011).
For the assay, ion mobility experiments were performed on the ACQUITY UPLC I-Class System and the SYNAPT G2-S using a series of standard solutions, spiked matrices, and the previously described proficiency test. Ion mobility has been successfully used to reduce false positives and false negatives in screening methods for pesticide residues in food. Ion mobility is a process which differentiates molecules as they traverse through a gas, and their progress is related to their average rotational collision cross section (CCS). The CCS is determined by very unique molecular properties, and therefore CCS itself is an important distinguishing characteristic of an ion which is related to its chemical structure (mass, size) and three-dimensional conformation (shape). The conformation can be influenced by a number of factors, including the number and location of charges.
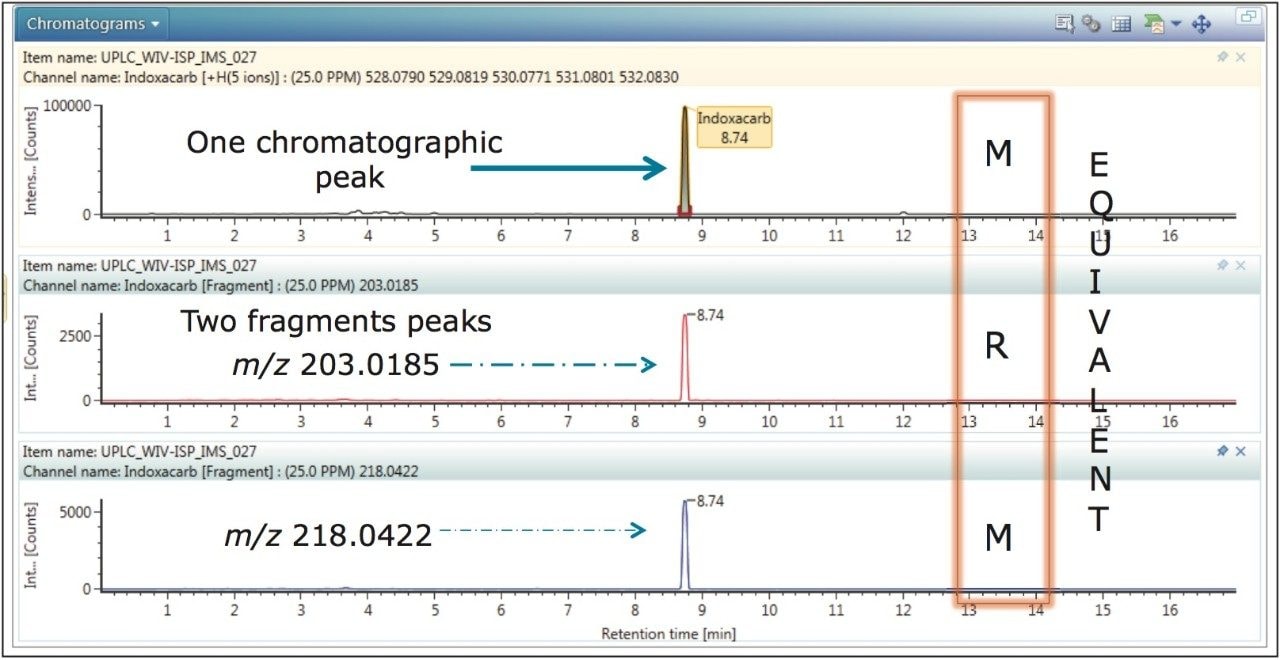
In Figure 2, indoxacarb has been selected from the identified components. The retention time aligned extracted mass chromatograms for the precursor ion and characteristic fragment ions of indoxacarb (determined to be present in proficiency sample FV-13) are presented. The data illustrated is representative of MRM transition data that would typically be acquired using a tandem MS platform, where the fragments identified at m/z 218 and m/z 203 could be selected as the confirmation MRM transitions of choice. These fragments have been correctly identified based upon fragmentation identification criteria within the UNIFI pesticide library used to perform a targeted screen. Confidence in the correct identification of indoxacarb could be justified, because the retention time, accurate mass, fragment ion, and CCS tolerances were met.
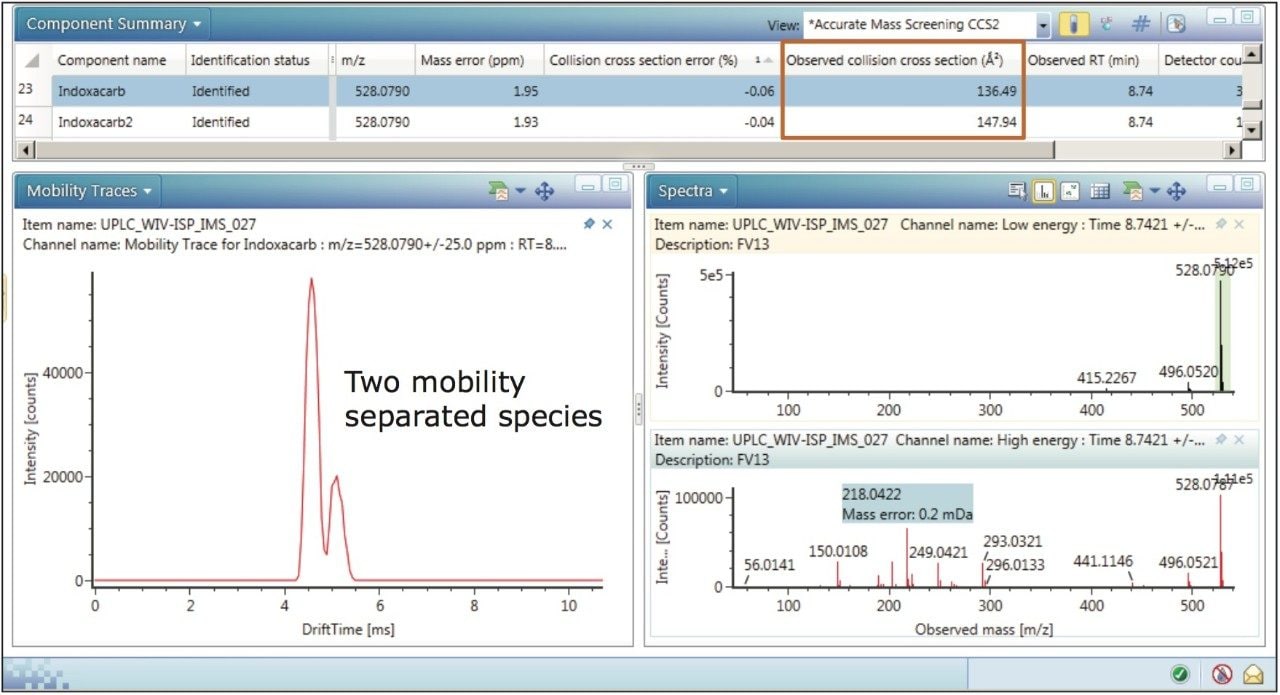
The mobility trace obtained, shown in Figure 3, indicates that indoxacarb is comprised of two mobility separated species, whereas the conventional precursor ion extracted mass chromatogram does not provide this information. From the structure of indoxacarb it can be seen that protonation is possible on more than one site. Multiple sites of protonation have been observed, where two protomers have been mobility resolved.3,4 The CCS values of 136.49 Å2 and 147.94 Å2 that were previously obtained and recorded can now be utilized to identify indoxacarb with the same level of confidence. All mobility species linked to the same target compound and incorporated in the target CCS list will enhance the screening performance of the method in future assays.
Presented in Figure 4 are the fragmentation spectra generated from the mobility separated protomers of indoxacarb. The distinctive fragments of each respective protomer are shown: a red diamond represents one protomer and a blue diamond represents the other, as well as the common fragments of each protomer (yellow circles). It has previously been shown that the ability to observe the protomer formation can be affected by experimental parameters such as flow rate, matrix, cone voltage and capillary voltage, and that these parameters would likely impact the reproducibility of those experiments as well as the fragments and their intensities observed.5 This in turn could also impact the assays where MRM transition is the method of choice for confirmatory analysis. It illustrates how critical the choice of MRM transition is and it also provides a unique insight into the fragmentation process being observed. The conventional fragmentation spectrum would be comprised of fragments from both protomers. If a protomer’s formation fluctuates, so will the expected fragments. Ion mobility with full spectra acquisition offers a route to greater understanding of the data generated as well as the ability to produce more robust assays.
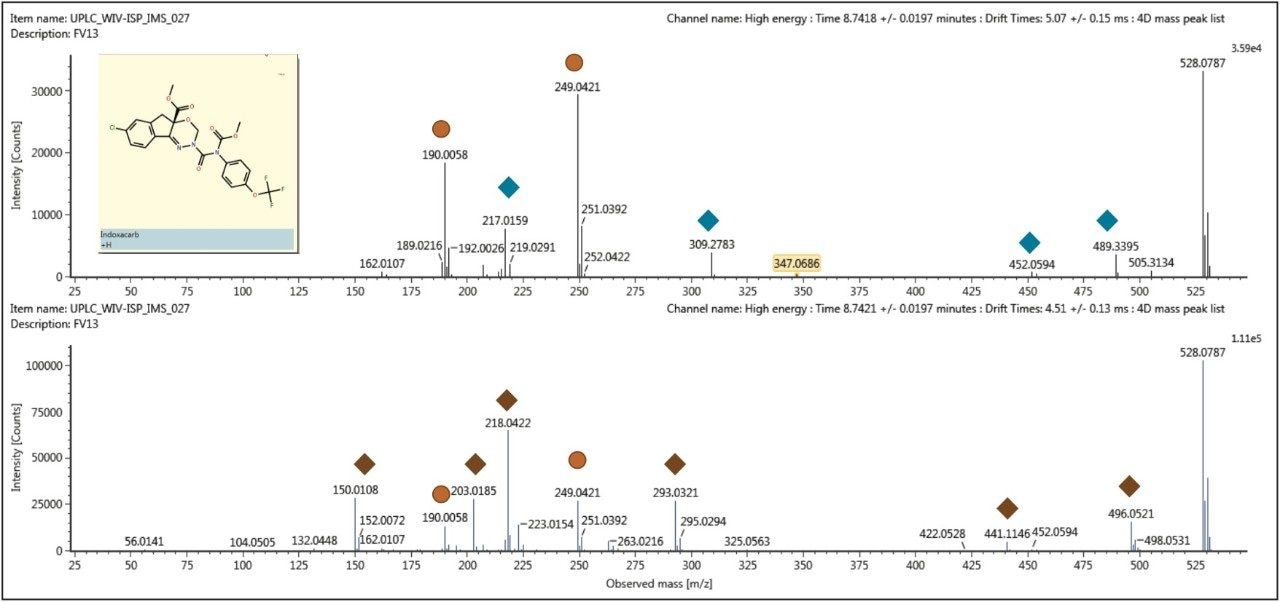
Using selective techniques such as FAIMS (Field Asymmetric Waveform Ion Mobility Spectrometry), development of robust assays would be a challenge. Because the number of compounds that can be targeted is limited by duty cycle, it would be difficult to optimize the parameters on an unknown ionized species. The data presented here was generated in a non-targeted analysis experiment, which enabled the pesticide protomers to be discovered. In the example shown of the indoxacarb protomers, it was possible to demonstrate that there are common fragments at m/z 190 and m/z 249.
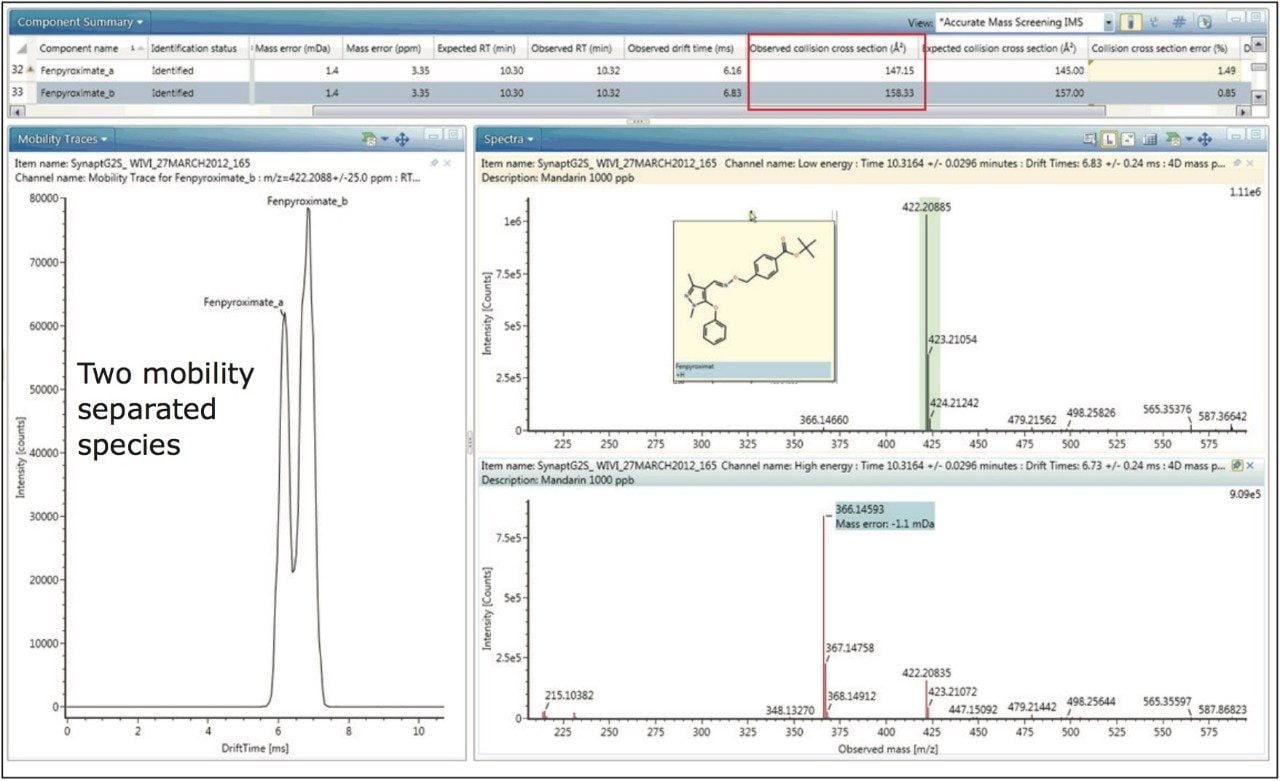
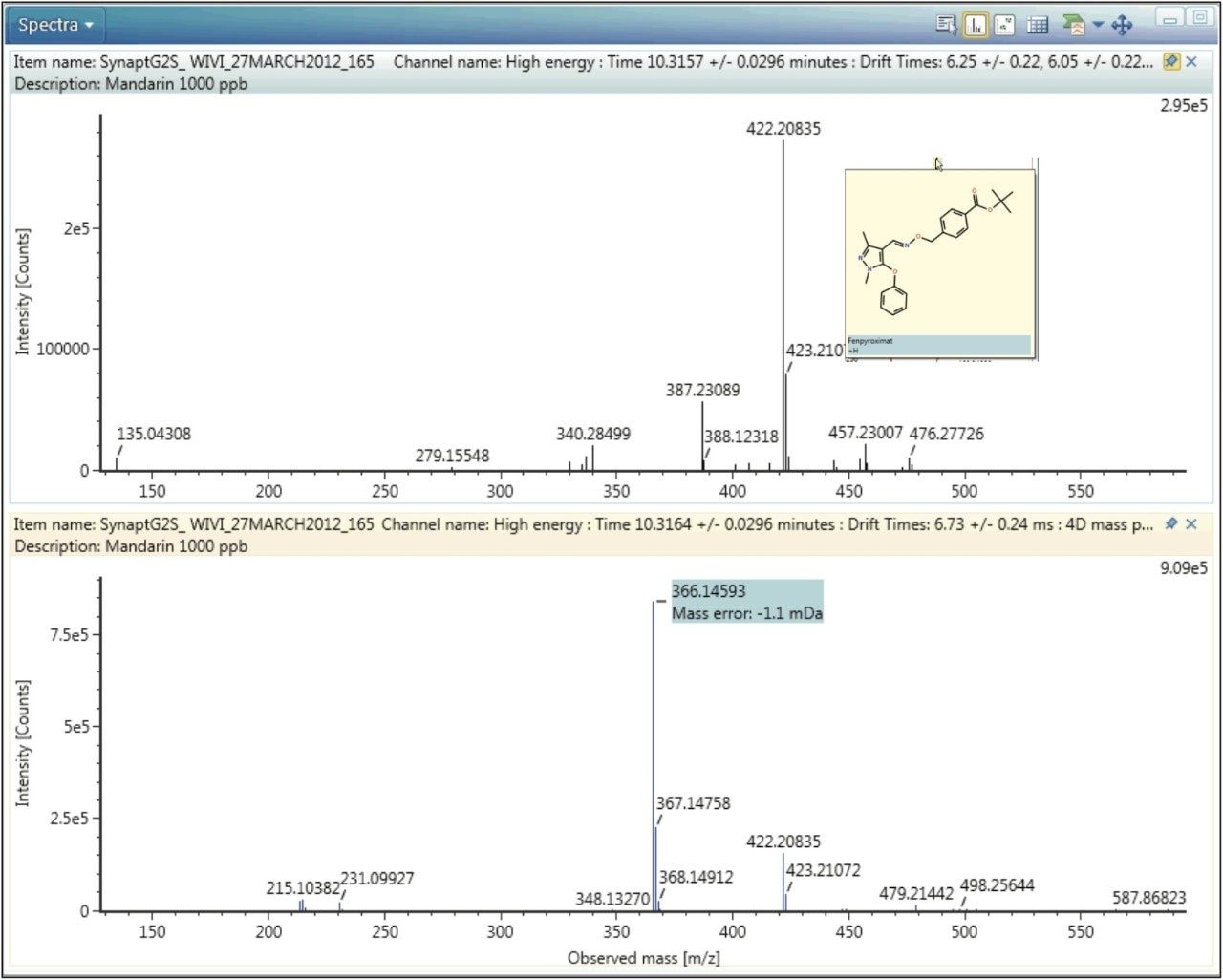
A further example of protomer formation in pesticide residue analysis screening is shown in Figure 5. Here, the mobility trace illustrating the observation of two protomers of fenpyroximate with measured CCS values of 147.15 Å2 and 158.33 Å2 is presented. The corresponding single component mobility resolved protomer fragmentation spectra are presented in Figure 6. Protomers of fenpyroximate each respectively produce one of the two most abundant fragment ions and once again, if conventional MRM analysis was performed, ion ratio measurement would be affected by protomer formation.
Within the context of initial ion mobility residue screening studies, a greater understanding of the analytical challenges has been obtained. The benefits of IMS-MS over non selective techniques such as FAIMS, are clearly illustrated in the case of IMS-MS, where ion mobility is acquired for all components regardless of the sample complexity. The benefits of using time-of-flight mass spectrometry for historical data review, are well known. This capability also applies to IMS-MS. The discovery and presentation of multiple sites of protonation occurring during the analysis for pesticide residues can only be possible if ion mobility data is acquired for all of the components in a sample. Further investigations are warranted, based upon these initial observations, to obtain a full understanding of the experimental parameters that cause protomer formation.
720005028, June 2014