
This is an Application Brief and does not contain a detailed Experimental section.
In this technology brief, vitamin A (retinyl acetate and retinyl palmitate) and vitamin E (alpha-tocopheryl acetate and alpha-tocopherol) were extracted from infant formula samples by a simple liquid-liquid extraction, then analyzed on a Waters ACQUITY UPC2 System coupled with a UPC2 PDA Detector. The separation of the analytes was achieved on a single Viridis HSS C18 SB 3.0 x 100 mm, 1.8 μm Column. UPC2 provides a faster, simpler, and “greener” approach for the simultaneous determination of vitamins A and E in infant formulas.
Determination of fat-soluble vitamins in food products typically involves lengthy sample preparation (such as saponification and extraction), followed by high performance liquid chromatography (HPLC) with UV or fluorescence detection.1 Vitamins A and E are often determined in the same analysis because of their similar sample preparation requirements.1 Recently, a simple sample preparation procedure without saponification was proposed in a simultaneous determination of vitamins A and E in infant formula (IF) method, where various forms of vitamins A and E were separated and quantified in a single injection.2 The elimination of saponification greatly increased the throughput of the analysis; however, the chromatography time was still lengthy (25 minutes), and the resolution of cis- and trans- forms of vitamin A were not evaluated in the paper.
Waters UltraPerformance Convergence Chromatography (UPC2) leverages the unique properties of supercritical carbon dioxide, including low viscosity, high diffusivity, and liquid-like solvation power. It provides an alternative approach to normal phase LC, reversed phase LC, and gas chromatography (GC) for a wide range of analytical challenges.3,4 To investigate the performance of UPC2 for the simultaneous determination of vitamins A and E, a feasibility study on commercial IF samples was performed.
In this feasibility study, vitamin A (retinyl acetate and retinyl palmitate) and vitamin E (alpha-tocopheryl acetate and alpha-tocopherol) were extracted from IF samples by a simple liquid-liquid extraction, then analyzed on an ACQUITY UPC2 System coupled with an ACQUITY UPC2 PDA Detector. The separation of the analytes was achieved on a single ACQUITY UPC2 HSS C18 SB 3.0 x 100 mm, 1.8 µm Column under a gradient elution of a carbon dioxide and methanol mixture (3% to 10% methanol). The chromatograms for these compounds were extracted from the PDA data at their maximum absorbance wavelength of 320 nm, 283 nm, 293 nm for retinol esters, alpha-tocopheryl acetate, and alpha-tocopherol, respectively, as shown in Figure 1.
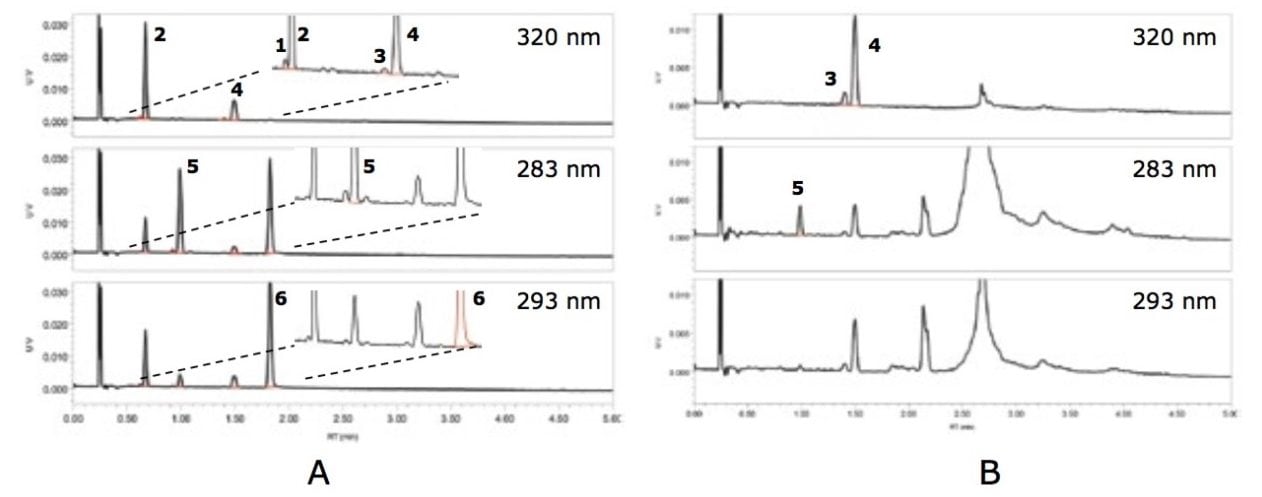
UPC2 provides a fast and well-resolved separation of vitamins A and E. All of the compounds, including the cis- and trans- forms of the retinol esters, were separated from each other, and were eluted before sample matrix peaks. The total cycle time, including column equilibration, was eight minutes per injection, which was at least three times faster than the typical run time (25 minutes) using other methods. Details of the method’s analytical performance in linearity, sensitivity, repeatability, and recovery are shown in Tables 1 and 2. Although the sample extract can be injected directly onto the system, the LOQ result indicated that evaporation or concentration of the extract may be needed for some compounds, depending on their content levels in IF samples. In this study, the sample extracts were concentrated ten-fold by evaporation for the vitamin E determination.
UPC2 is an environmentally friendly, or “green” technology. The source of the primary mobile phase, carbon dioxide, is recaptured carbon dioxide that is released from other industries, so the use of carbon dioxide does not generate any new greenhouse gases. The co-solvent (methanol) consumption was only 0.9 mL for each injection by UPC2 compared to 10 mL of hexane used in the method in reference 2. This corresponds to at least a 90% reduction in solvent consumption.
Simultaneous determination of cis- and transretinyl palmitate, cis- and trans- retinyl acetate, alpha-tocopheryl acetate, and alpha-tocopherol in commercial IF samples was achieved in a single injection using a single Viridis Column on Waters’ ACQUITY UPC2 System with PDA detection.
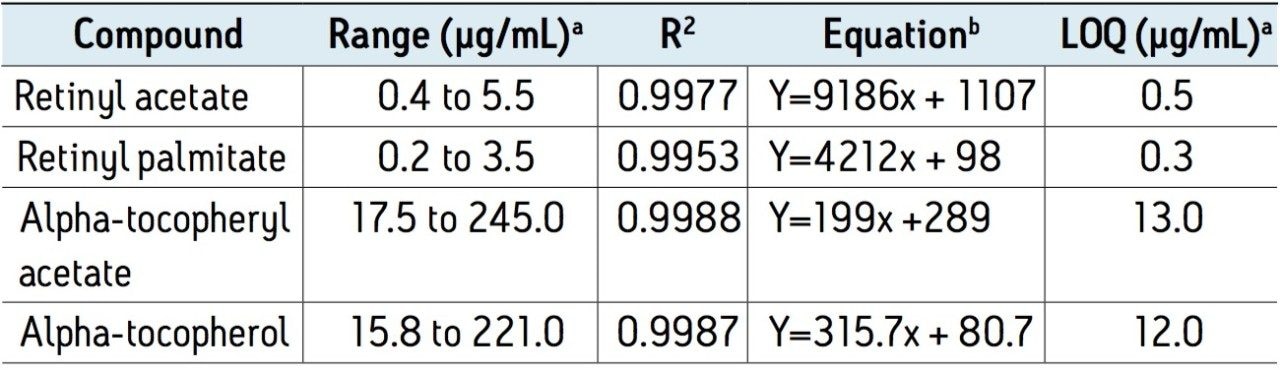
Table 1. Linearity of the method and estimated LOQ by UPC2/PDA.
a Expressed in µg of analyte per mL in standard solution.
b Y, peak area; x, concentration (µg/mL).

Table 2. Repeatability and recovery result obtained on spiked infant formula samples.
a The values are expressed in µg vitamin per gram of infant formula powder.
The sample analysis time took eight minutes, three times faster than a typical analysis time, and the solvent consumption for each injection was 0.9 mL, one tenth of that in a normal phase LC method. This approach provides promising results in resolution, linearity, sensitivity, precision, and accuracy. UPC2 has great potential to become a practical solution for the routine determination of vitamins A and E in infant formula products.
720004538, January 2013