In this study, a compendial assay method for Telmisartan Tablets written in the United States Pharmacopeia (USP) was transferred from HPLC to UPLC using sub-2-μm particle technology with reduced run time and narrower peak shape for better chromatography.
Telmisartan is a non-peptide angiotensin II receptor blocker (ARB) used to treat high blood pressure, to help prevent strokes and heart attacks. It is occasionally used to treat congestive heart failure or a condition in which the heart is unable to pump enough blood to the rest of the body, and to help protect kidneys from damage due to diabetes.1 Telmisartan is available as tablets for oral administration, containing 20, 40 or 80 mg of Telmisartan. It is marketed under the trade name Micardis by Boehringer Ingelheim Pharmaceutical, Inc. and is also available in a generic form.
In this study, a compendial assay method for Telmisartan Tablets written in the United States Pharmacopeia (USP)2 was transferred from HPLC to UPLC using sub-2-μm particle technology with reduced run time and narrower peak shape for better chromatography. The routine use of the method was conducted over 3000 injections using standard and sample preparations. The goal of transferring methodology to UPLC is to provide cost reductions for manufacturing and improvements in method robustness for routine analysis.
|
LC System: |
Alliance HPLC with 2489 UV/Visible detector |
|
Column: |
XSelect HSS T3, 4.6 x 50 mm, 5 μm (USP designation: L1), part number 186004794 |
|
Mobile Phase: |
70:30 methanol:buffer |
|
Buffer: |
17 mM ammonium dihydrogen phosphate in water, pH 3.0 adjusted with 1 M phosphoric acid |
|
Wash Solvents: |
70:30 methanol:water |
|
Separation Mode: |
Isocratic |
|
Flow Rate: |
0.7 mL/min |
|
Column Temp.: |
40 °C |
|
Sample Temp.: |
8 °C |
|
Injection Volume: |
5.0 μL |
|
Run Time: |
6.0 minutes |
|
Detection: |
UV at 298 nm |
|
LC System: |
ACQUITY UPLC with PDA detector |
|
|
Column: |
ACQUITY UPLC HSS T3, 2.1 x 30 mm, 1.8 μm (USP designation: L1), part number 186003944 |
|
|
Mobile Phase: |
70:30 methanol:buffer |
|
|
Buffer: |
17 mM ammonium dihydrogen phosphate in water, pH 3.0 adjusted with 1 M phosphoric acid |
|
|
Wash Solvents: |
Weak wash (600 μL): 70:30 methanol:water Strong wash (200 μL): 95:5 methanol:water |
|
|
Separation Mode: |
Isocratic |
|
|
Flow Rate: |
0.41 mL/min |
|
|
Column Temp.: |
40 °C |
|
|
Sample Temp.: |
8 °C |
|
|
Injection Volume: |
0.6 μL |
|
|
Run Time: |
1.5 minutes |
|
|
Detection: |
UV at 298 nm |
|
|
Data Management: |
Empower 2 CDS |
For 5 replicate injections
Stock standards, working standards, and sample solutions were prepared as per the assay method defined in the USP Monograph for Telmisartan Tablets.
Stock standard was prepared by dissolving an accurate amount of Telmisartan reference material and Telmisartan Related Compound A RS in 0.005N sodium hydroxide in methanol to make a solution at approximately 0.8 mg/mL and 0.1 mg/mL, respectively. The stock solution was then diluted with mobile phase to a working concentration of approximately 0.11 mg/mL of Telmisartan and 0.013 mg/mL of Telmisartan Related Compound A, respectively.
Micardis (telmisartan) 20 mg Tablets manufactured by Boehringer Ingelheim were used in this study. Tablets were dissolved in 0.005N sodium hydroxide in methanol via sonication for 20 minutes to obtain 0.8 mg/mL stock solutions. The stock solutions were then diluted with mobile phase to 0.11 mg/mL concentration.
The USP monograph for Telmisartan Tablets specifies an isocratic separation at 0.7 mL/min to deliver a mobile phase containing 70:30 methanol:ammonium dihydrogen phosphate buffer. Standard and sample solutions are injected with an amount of 5 μL on a column with dimensions of 4.6 mm x 50 mm, 5 μm particle size and L1 packing. Column temperature is maintained at 40 °C. The experiment was collected and analyzed at a wavelength of 298 nm.
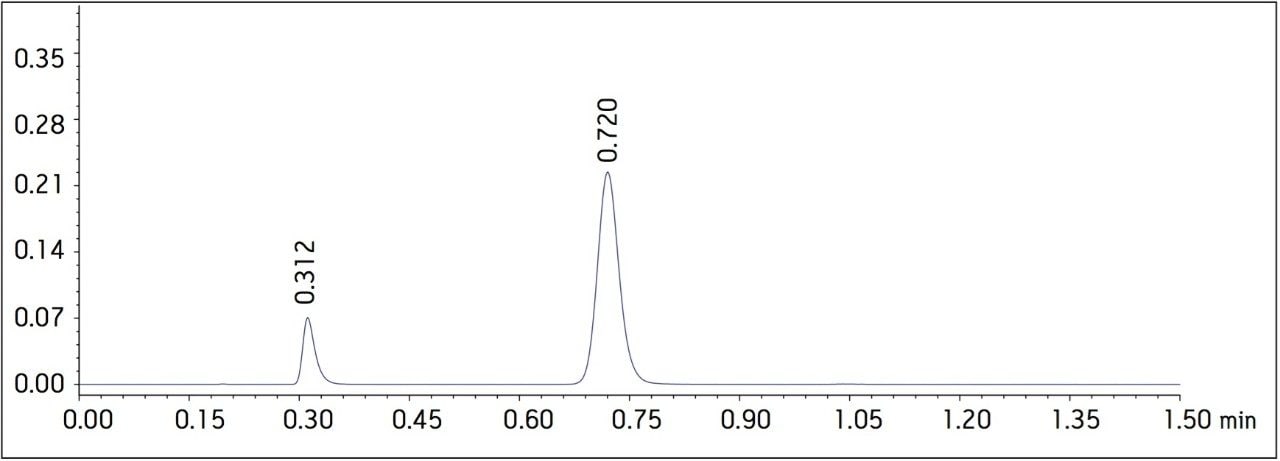
The USP methodology for Telmisartan Tablets was tested, as written, using an Alliance HPLC system and a Waters L1 column selected using the Waters Column Selectivity Chart. The HPLC analysis was performed with a Waters HSS T3 column (4.6 x 50 mm, 5 μm). The HPLC methodology was transferred to UPLC using the Waters ACQUITY UPLC Columns Calculator as described in the application note 720003721EN.3 The resulting method was run on ACQUITY UPLC instrumentation, producing narrower peaks and reduced analysis time. A comparison of Telmisartan Standard Solution acquired using Alliance HPLC and ACQUITY UPLC systems is displayed in Figure 1. In summary, system suitability requirements for the Telmisartan Tablets on the Alliance HPLC and the ACQUITY UPLC systems passed the USP criteria for the assay method and are summarized in Table 1.
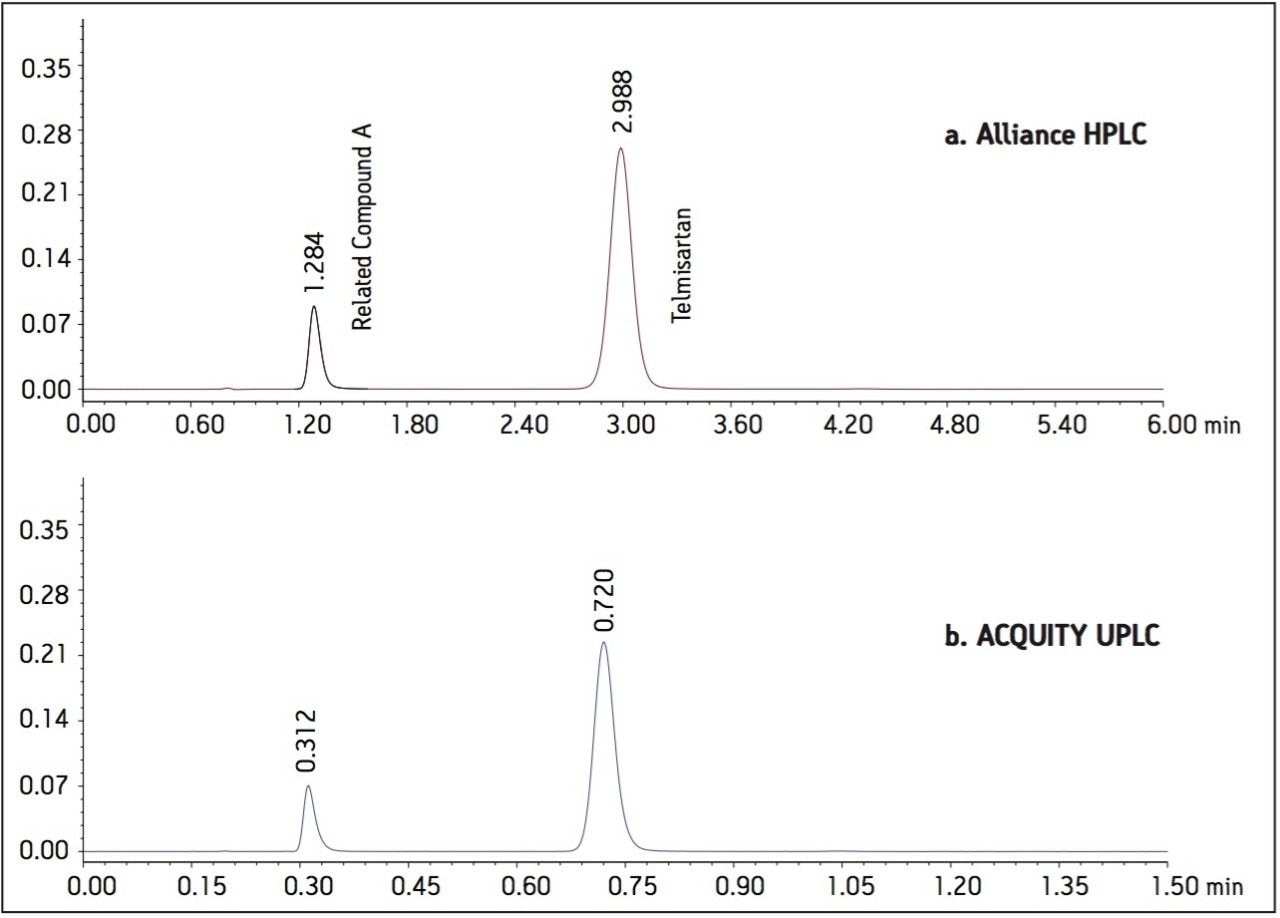
Figure 1. Telmisartan standard solution
a. Acquired using an Alliance HPLC system with an XSelect HSS T3 4.6 x 50 mm, 5 μm column.
b. Acquired using an ACQUITY UPLC system with an ACQUITY UPLC HSS T3 2.1 x 30 mm, 1.8 μm column.
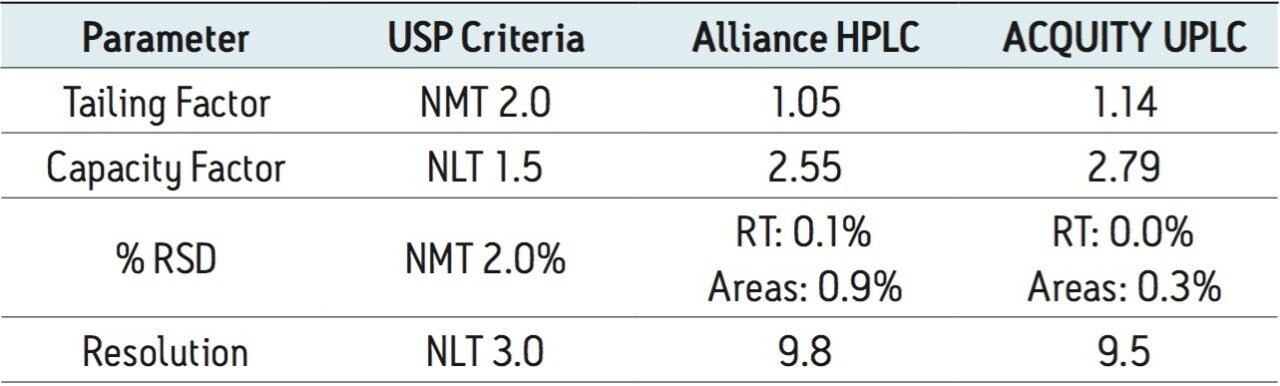
The routine use study of the UPLC assay method for Telmisartan Tablets was conducted after completion of the method transfer to demonstrate that the method is suitable for long-term use within a quality control laboratory. The goal of the study was to evaluate the system performance, column behavior and robustness of the transferred method over 3000 injections of standard and tablet preparation solutions.
Four separate sample preparations proved to have good reproducibility of the sample preparation procedure. The sample set consisted of 2 injections of blank and 8 injections of sample solution, bracketed with two replicate injections of a Telmisartan standard. The data obtained for the Telmisartan Tablet sample solution is shown in Figure 2. The system suitability parameters for the 5 replicate injections of standard monitored during the course of the study are summarized in Table 2.
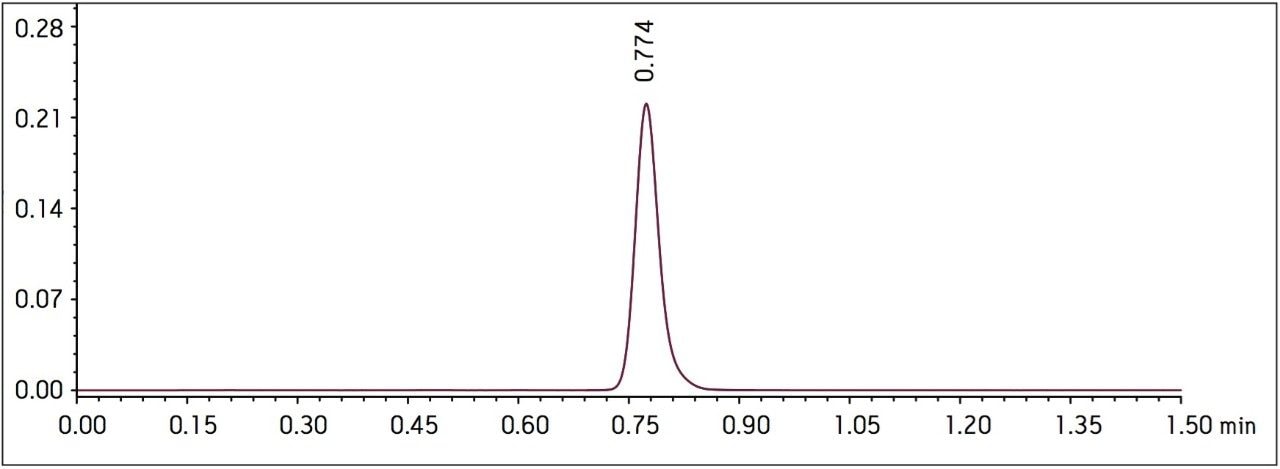
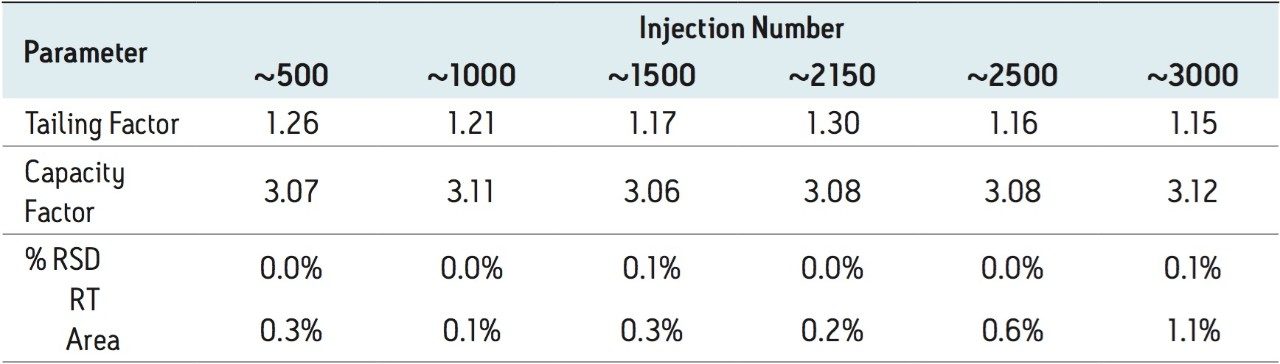
Method performance was excellent for the first 2000 injections. At approximately 2100 injections, some tailing of the Telmisartan peak was observed but still well within the system suitability requirements. Pressure increased slightly, however washing the column reduced system pressure and significantly improved peak tailing. Overall, system pressure remained stable throughout the course of the study (Figure 3). In summary, all of the USP system suitability criteria are still acceptable after 3000 injections onto the ACQUITY UPLC column, including resolution between Telmisartan and Telmisartan Related Compound A as displayed in Figure 4.
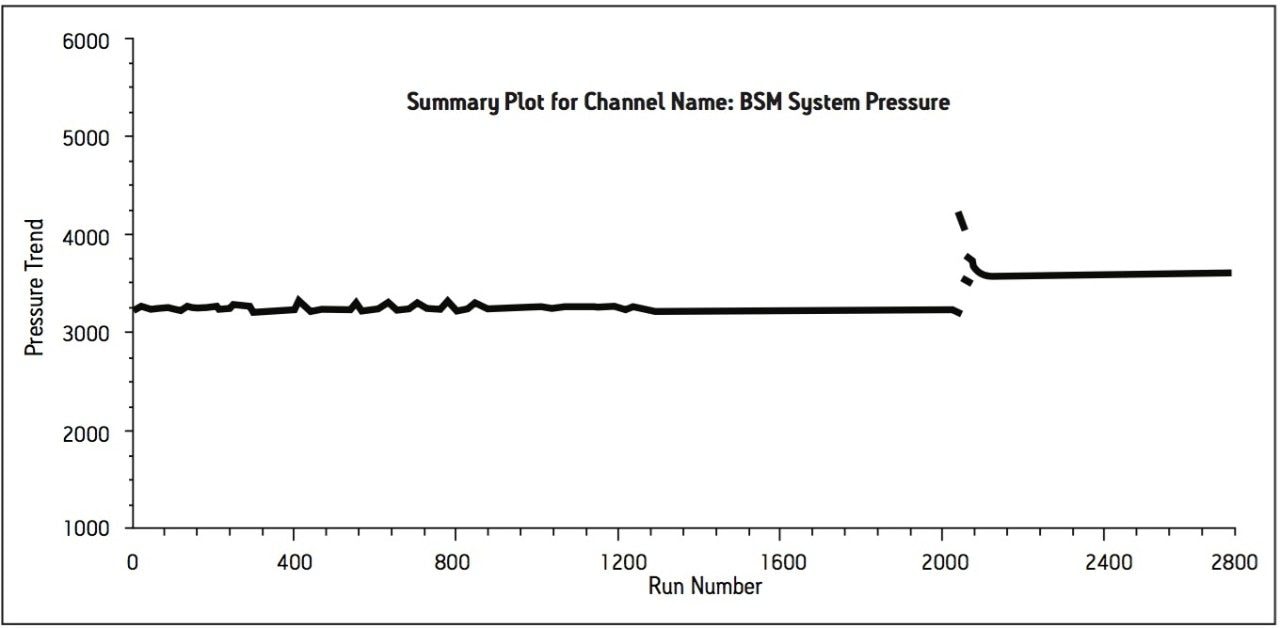
The transfer of this compendial method proves applicability of successfully transferring existing USP methods from HPLC to UPLC. The resulting UPLC method provides a 75% reduction in run time while maintaining the integrity of the system suitability specifications of the USP method. Furthermore, the amount of mobile phase used per injection is 0.6 mL compared to 4.2 mL for the HPLC method, which is an 86% reduction in mobile-phase consumption per injection. The other benefit through this transfer is an 88% reduction in sample consumption. Overall, the resulting UPLC method provides cost saving for solvent and waste disposal. Moreover, approximately 3000 injections were performed onto the ACQUITY UPLC column with no significant increase in system pressure and decrease in method performance. Column washing was applied after 2000 injections to reduce peak tailing and system pressure. This cleaning regenerated and returned the column to its original operating conditions. The ACQUITY UPLC column demonstrated excellent performance for over 3000 injections, which is important for a long-term routine use in a quality control laboratory.
720004133, April 2013