This is an Application Brief and does not contain a detailed Experimental section.
We demonstrate the utility of electron transfer dissociation (ETD)-MS for the provision of sequence-specific information in investigating weak peptide-metallodrug interactions that could not be obtained using collision-induced dissociation (CID).
ETD-MS is a powerful technique as a probe for identifying weak peptide-anticancer drug interactions that may affect pharmacology and efficacy.
Currently the Pt-based drugs cisplatin, cis- [PtCl2(NH3)2], carboplatin, and oxaliplatin are widely used in cancer chemotherapy. The clinical use of cisplatin, however, has a number of drawbacks, including neurotoxicity, nephrotoxicity, acquired or inherent resistance in some cancer cells, and a limited spectrum of activity against different types of cancer. For these reasons, the potential of other transition metal-based agents, such as anticancer agents, is actively being explored. For example, promising organoruthenium complexes of the type [(n6-arene)RuII(en)Cl]+ (arene = eg: biphenyl or tetrahydroanthracene; en= ethylenediamine), show promising in vitro and in vivo activity, including towards cisplatin-resistant cell lines. These complexes have a “piano-stool” type geometry and, unlike cisplatin which has two reactive Pt-Cl coordination bonds, they have only one reactive site (Ru-Cl). To gain an understanding of the mode of action of anticancer metallodrugs it is important to study their interaction with biomolecules, with which they could potentially react. These include amino acids, peptides, proteins, oligonucleotides, and DNA.
Here we report the application of two tandem MS approaches, CID and ETD, for the rapid determination of the binding sites of [(n6-biphenyl)RuII(en)Cl]+ upon the neurotransmitter Substance-P.
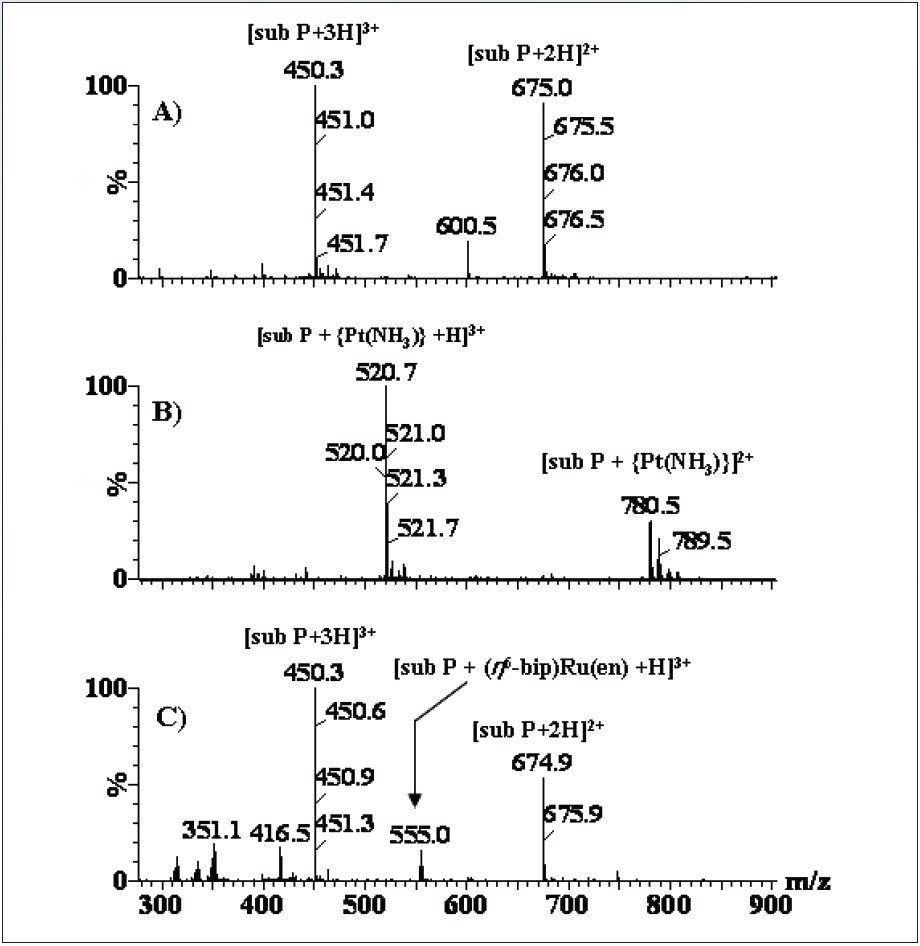
First, we demonstrate the mass spectra obtained by reacting Substance-P with cisplatin [PtCl2(NH3)2] and [(n6-biphenyl)RuII(en)Cl]+, as shown in Figure 1. These data suggest that the binding of {(n6-bip)Ru(en)}2+ to Sub-P is relatively weaker than binding to cisplatin. In order to precisely determine the Ru-based drug binding site to Substance-P, we utilized both CID and ETD for sequence-specific information.
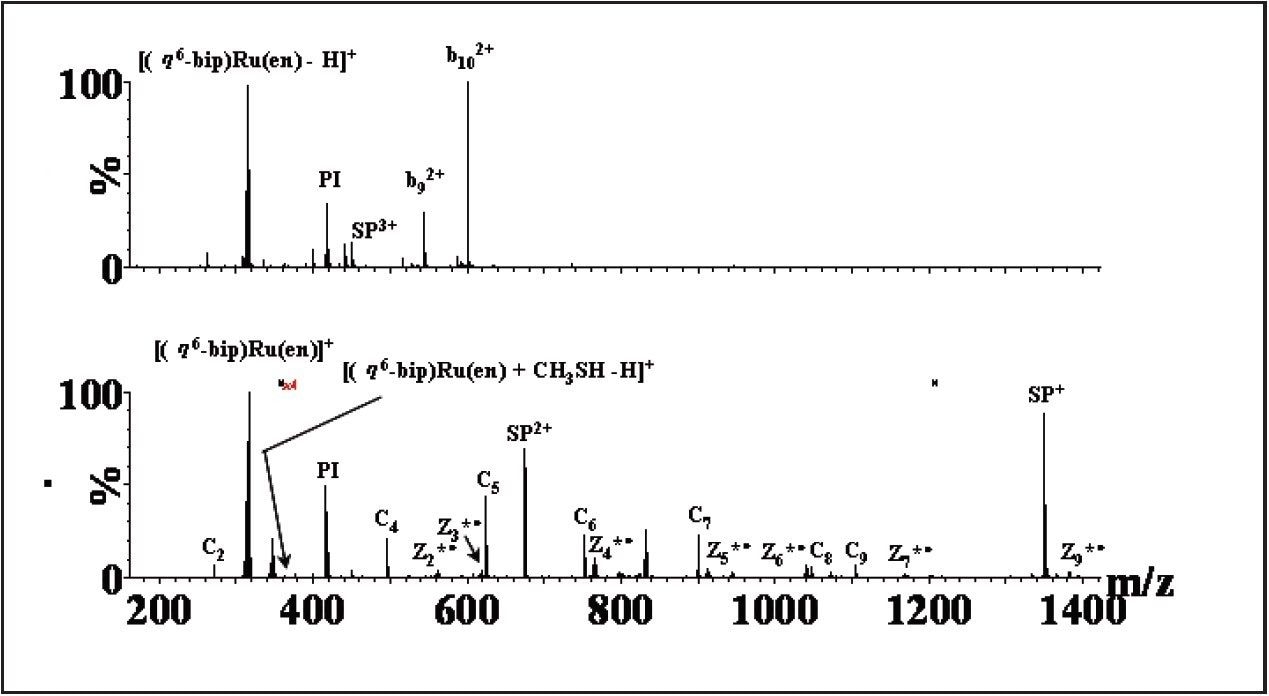
ETD is a radical-driven fragmentation technique and results in cleavage of the peptide N-Cα bond to give c and z• peptide fragment ions (cf. b and y" ions using CID) and has been shown to be particularly useful for determining sites of labile modifications.
Figure 2 shows a comparison of the tandem MS data. Upon CID of the selected quadruply-charged precursor ion, no sequence-specific ions containing the Ru drug were observed, as seen in Figure 2(A). The only peak containing Ru was detected at m/z 315, [{(n6-bip) Ru(en)} -H]+ resulting from loss of bound Sub-P. In contrast the ETD-generated mass spectrum, shown in Figure 2(B), provided identification of the drug binding site. In the ETD experiment, the precursor ion, [Sub-P + {(n6-bip)Ru(en)} +2H]4+ was allowed to interact with anions produced from nitrosobenzene (m/z 107), thus producing cn + and z*n + type ions (* represents modification by the Ru-drug, i.e., [{(n6-bip)Ru(en)}]2+).
It is known that within 24 hours, 65% to 90% of administered cisplatin, for example, is bound to proteins in blood plasma. Such platinum-protein interactions reduce the level of excreted Pt and increase its deposition in tissues. These reactions may contribute to toxic side-effects in patients, and also to the mechanism of cancer cell toxicity. This work highlights the usefulness of the ETD-MS technique as a probe for identifying weak peptide-anticancer drug interactions, which can be used as a tool for studying interactions with plasma proteins that may affect drug pharmacology and efficacy.
Waters Corporation Manchester, UK, would like to acknowledge Professor Peter Sadler and Dr. Abraha Habtemariam of University of Warwick, UK.
720003583, June 2010