
Currently, there are 84 known mutations of human transthyretin. Of these 84 mutations, one (lle68 to Leu) has no mass difference while six differ by a single amu. All other variants show a mass difference of ten or more amu from normal transthyretin. From the current list, 92% of all possible mutations would therefore be identified by this on-line mass spectral analysis. The exact identity would require further analysis since many are isobaric.
Familial transthyretin amyloidosis is an inherited systemic disease in which protein deposits ultimately lead to organ failure(s) and death. Familial transthyretin amyloidosis is associated with genetic variants of transthyretin in which single amino acid substitutions have been found in the primary structure. Transthyretin is a plasma transport protein that exists as a monomer of mass 13761 amu containing 127 amino acids. It circulates as a tetramer in plasma and has unique binding sites for both thyroxine and retinol binding protein. One recent count indicated 84 genetic variants, the vast majority being amyloidogenic.1 Screening for known variants and identification of unknown variants poses a unique set of problems. In response to some of these issues, we developed an on-line assay for transthyretin genetic variants that utilizes an immunoaffinity column to purify transthyretin directly from 20 µL of serum. The immunopurified transthyretin is desalted on a reversed-phase trap and eluted into the electrospray source of a mass spectrometer for analysis. Transformed spectra of the resulting multiply charged spectra clearly indicate the presence or absence of transthyretin genetic variants. On all analyses to date, perfect correlation has been found between actual DNA sequence analysis and mass spectral results. The results are obtained in less than twenty minutes and are easily automated providing a rapid assay for screening for common genetic variants.
Serum samples are filtered through a 25 mm syringe filter (Acrodisc 0.8/0.2 µm Supor, Pall Corp. Ann Arbor, Ml) prior to use and then diluted 1:1 with phosphate buffered saline, pH 7.4. Forty microliters of this diluted serum was injected.
A Waters Micromass Quattro LC Mass Spectrometer operated in positive ion mode was equipped with a standard ZSpray source. The source temperature and desolvation temperature were 90 °C and 150 °C respectively. Cone voltage and capillary voltage were 40V and 3.3kV respectively. Cone gas flow was 51 L/hr and desolvation gas flow was 483 L/hr. Two-second continuous scans from 600 to 2200 amu with a two-second interscan delay were utilized. Scans containing the eluted transthyretin were summed and processed.
The chromatography system was a Waters Alliance HT System (50 µL/min; isocratic, A: Phosphate Buffered Saline) with auxiliary fluid delivery using a Waters 600 pump (50 µL/min; isocratic, B: 100 mM glycine, pH 2.5) and two LC-10ADvp pumps with a SCL-10Avp controller (50 µL/min; C: 98/2 water/acetonitrile with 0.2% formic acid, D: 5/95 water/acetonitrile with 0.2% formic acid). The Waters Alliance HT System was controlled by Masslynx Software v3.4.
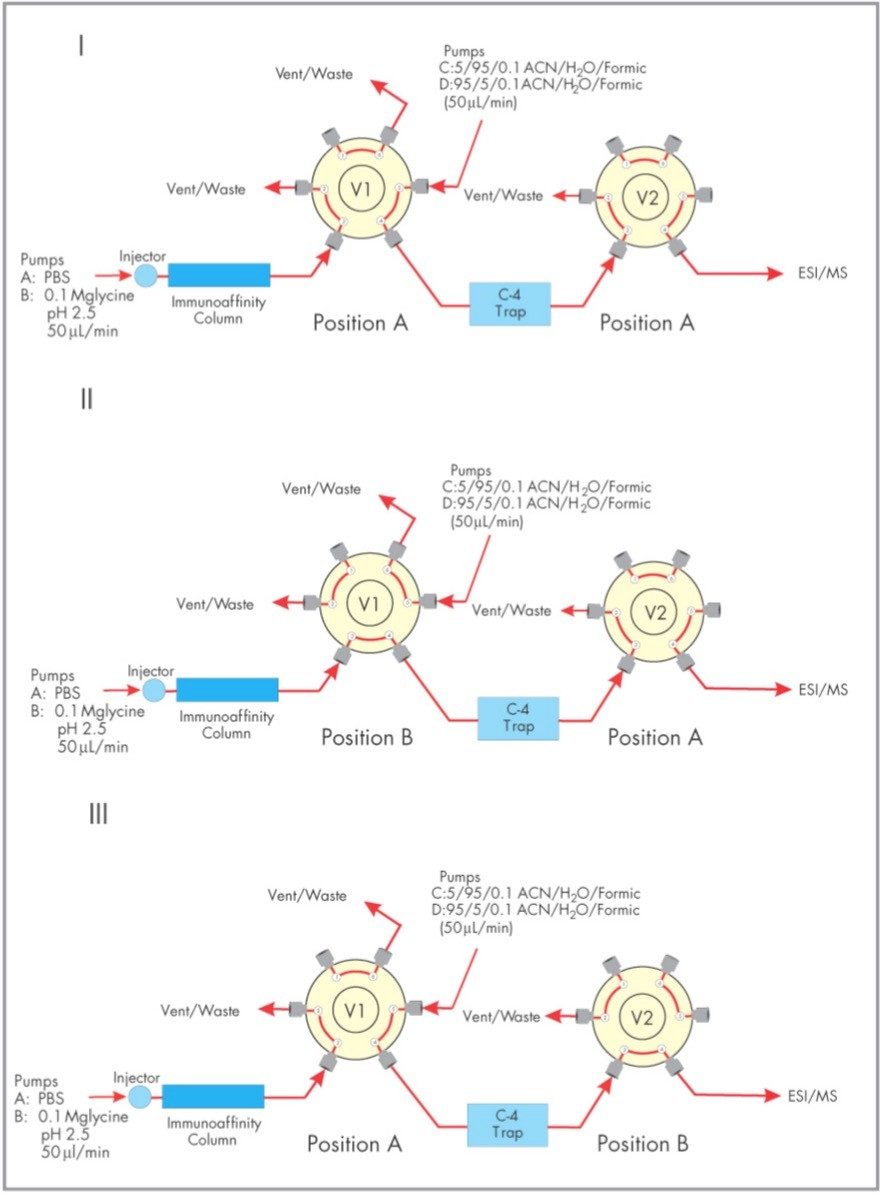
Three automated two-position valves (Rheodyne L.P., Rohnert Park, CA) were controlled with the contact closure outputs of the Waters 2790 Separations Module.
Rabbit antihuman transferrin antibodies (Dako Corp., Carpintera, CA) were coupled to POROS AL media utilizing the "recycling method" as per the manufacturer's instructions. Individual microbore guard columns (1 x 20 mm PEEK, Upchurch Scientific, Oak Harbor, WA) were packed with the anti-transthyretin POROS media (∼17 µL bed volume) . Binding buffer was 10 mM phosphate
pH 7.4, 150 mM NaCI. Elution buffer was 0.1 M glycine pH 2 .5. Bound transthyretin was washed with binding buffer for six minutes prior to elution with elution buffer (step gradient) for three minutes.
A 1 x 20 mm PEEK microbore Guard Column packed with 40 µm C4 silica (Bakerbond Prepscale WP Butyl (C4), J.T. Baker Research Products, Mallinckrodt Baker, Inc., 222 Red School Lane, Phillipsburg, NJ) was utilized to desalt the immunopurified transthyretin prior to MS analysis. Elution of the desalted protein from this trap was via an organic gradient utilizing the C/D pumps. The gradient program was 2% D for three minutes (desalts bound transthyretin), two minute linear gradient to 95% D (elutes transthyretin), three minutes at 95% D, one minute linear gradient to 2% C (return to starting conditions), Stop.
One two-position valve is positioned prior to the affinity column and is plumbed to switch between binding buffer (A: PBS) and elution buffer (B: 0. 10 M glycine, pH 2.5). Another two-position valve V1 is plumbed to allow effluent from the affinity cartridge to flow to waste during the binding and washing periods and to the C4 trap during elution of bound transthyretin. The valve V2 is used as a divert valve to divert buffer salts from the ESI source.
Briefly, at time zero a sample is injected. Transthyretin binds to the affinity cartridge while valve V1 diverts unbound serum components to waste leaving transthyretin bound to the affinity cartridge. At t=6 minutes valve V1 diverts affinity column effluent onto the C4 trap. The transthyretin bound is released by switching to elution buffer B (6–9 minutes) and the eluted transthyretin is trapped on the C4 cartridge. Valve V1 returns (9 minutes) to its original position diverting the affinity cartridge effluent to waste allowing pumps C/D to pump across the C4 trap. Valve V2 diverts the first three minutes of the organic gradient (100% C, 9.1–12 minutes) to waste preventing any buffer salts from entering the source. At t=12 minutes valve V2 takes effluent from the C4 trap into the ESI source. A two minute gradient to 100% D (12–14 minutes) elutes transthyretin (16 minutes) from the C4 trap. The gradient stays at 100% D ( 14-17 minutes) before coming back to 100% C (17–18 minutes).
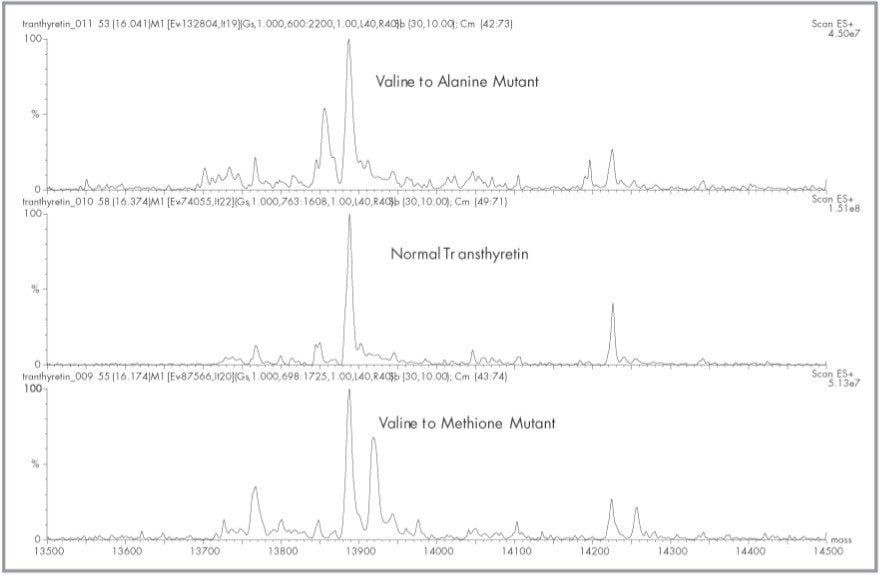
The schematic of Figure 1 indicates the connectivity and components of the HPLC pumps, immunoaffinity column, C4 trap, valves (Vl and V2) and the Micromass Quattro lC ESI Mass Spectrometer. The Waters Alliance HT HPLC System is controlled directly via the Masslynx Software. Contact closure outputs from the 2790 Separations Module control all valve positions and initiate the organic gradient which elutes transthyretin off the C4 reversed-phase trap. The complete automated analysis and recovery to initial conditions of both the immunoaffinity column and C4 trap is less than 20 minutes. The multiply charged spectra of transthyretin from a normal and valine to methionine mutant is shown in Figure 2. The multiply-charged spectra have been transformed onto a true mass scale using MaxEnt processing in Figure 3. No mathematical artifacts are generated by the MaxEnt processing.
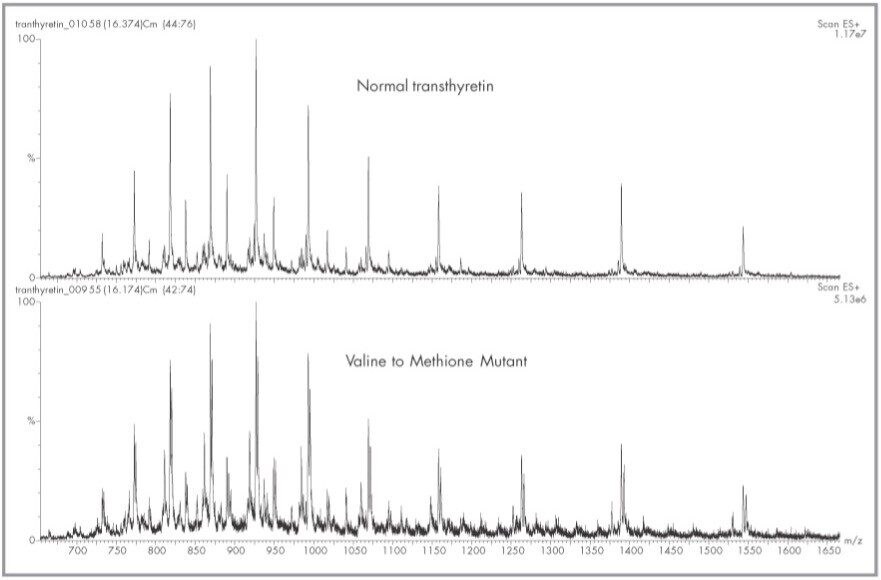
Interestingly, transthyretin circulates as free transthyretin, but its most abundant form circulates linked to free cysteine through a disulfide bond.2 Free transthyretin monomer has a theoretical average mass of 13 ,761.4 Do while that of the adduct is 13,880.6. The antibody binds to both forms, and both forms are seen in Figure 3. Adducts of cysteinylglycine and glutathione have also been reported.2 Our samples did not contain significant amounts of either cysteinylglycine or glutathione adducts. Interestingly, adducts did appear which seems to correlate with an adduct species of mass 458 amu. The identity of this adduct was not determined, but we have found it in all our samples analyzed to date. Only some samples appear to contain cysteinylglycine adducts, while the major species in all is the cysteine adduct.
Consistent with the work of others, the concentration of the abnormal transthyretin variants are considerably less than that of normal transthyretin.3
Currently, there are 84 known mutations of human transthyretin.1 Of these 84 mutations, one (lle68 to Leu) has no mass difference while six differ by a single amu. All other variants show a mass difference of ten or more amu from normal transthyretin. From the current list, 92% of all possible mutations would therefore be identified by this on-line mass spectral analysis. The exact identity would require further analysis since many are isobaric.
The capability to quickly and easily identify the presence of a transthyretin genetic variant by an online analysis should not be overlooked as a diagnostic for familial transthyretin amyloidosis. Our results were obtained on less than 25 µL of serum, but the procedures could be altered to accommodate larger or smaller sample sizes with the concomitant sensitivity changes.
Although there has been mention of the difficulty in this analysis due to the low variant to wild type ratios we had no problems in correctly identifying the presence of variants in a set of fourteen samples which contained known, unknown and normal transthyretin.4 DNA sequence data has subsequently confirmed all of our identifications.
Since all forms of the protein (free, cysteinylated, S-sulfated, etc.) that the anti-transthyretin antibodies bind to are seen in the mass spectra, all posttranslational modifications should be readily seen. If structural modifications are associated with the pathogenesis of tranthyretin amyloidosis, the ability to visualize these modifications will prove to be of great significance.
The 458 amu adduct we have seen in all our samples has not been previously reported and is currently under investigation.
The immunoaffinity cartridge utilized for the analysis is extremely robust and the same cartridge has been in use for all analyses to date. Similar cartridges have survived over 1000 injections without loss of bindings. Therefore the system is robust and with appropriate modifications, the entire assay could be performed in less than 10 minutes.
The online technique described here has the potential to become the assay of choice to screen for genetic variants of transthyretin and to elucidate structural characteristics that could prove useful in determining the pathology of this disease. The same basic procedures we utilized to diagnose carbohydrate deficient glycoprotein syndrome (CDGS) are utilized here.6,7 It is the authors impression that this is a robust platform that will have wide spread diagnostic applications that can be modified by simply changing the specificity of the immunoaffinity cartridge.
720000731, March 2004