

The journey of a GLP-1 receptor agonist (GLP-1 RA) molecule is complex. From discovery to quality control, and ultimately the delivery of safe, high-quality GLP-1 RA drugs, each stage in the molecule’s journey presents unique challenges.
Waters comprehensive workflow solutions are built to simplify this journey. By delivering robust, reproducible methods, minimizing analytical uncertainty, and accelerating development timelines, Waters helps you navigate every critical step with greater speed and confidence so your molecule can reach patients faster and safer.


Guidebook: Analysis and Characterization of GLP-1 Receptor Agonists
GLP-1 RAs are widely prescribed to treat type 2 diabetes and obesity. As demand increases, drug manufacturers need proven, reliable methods to ensure product safety and efficacy
Explore our guidebook for Waters comprehensive review of advanced analytical technologies designed to support critical quality attribute (CQA) analysis of GLP-1 RAs.
GLP-1 RAs are widely prescribed to treat type 2 diabetes and obesity. As demand increases, drug manufacturers need proven, reliable methods to ensure product safety and efficacy
Explore our guidebook for Waters comprehensive review of advanced analytical technologies designed to support critical quality attribute (CQA) analysis of GLP-1 RAs.

Analyze samples and generate meaningful results effortlessly with Waters Empower Software Solutions, the industry-leading easy-to-use chromatography data system that helps you minimize training, eliminate manual labor, and increase productivity.
Generate high resolution mass spectrometry data with waters_connect Informatics Platform, an app-based, compliant-ready environment to manage multiple systems on a single network, enable method sharing and data review with the scalability to meet your future lab requirements.
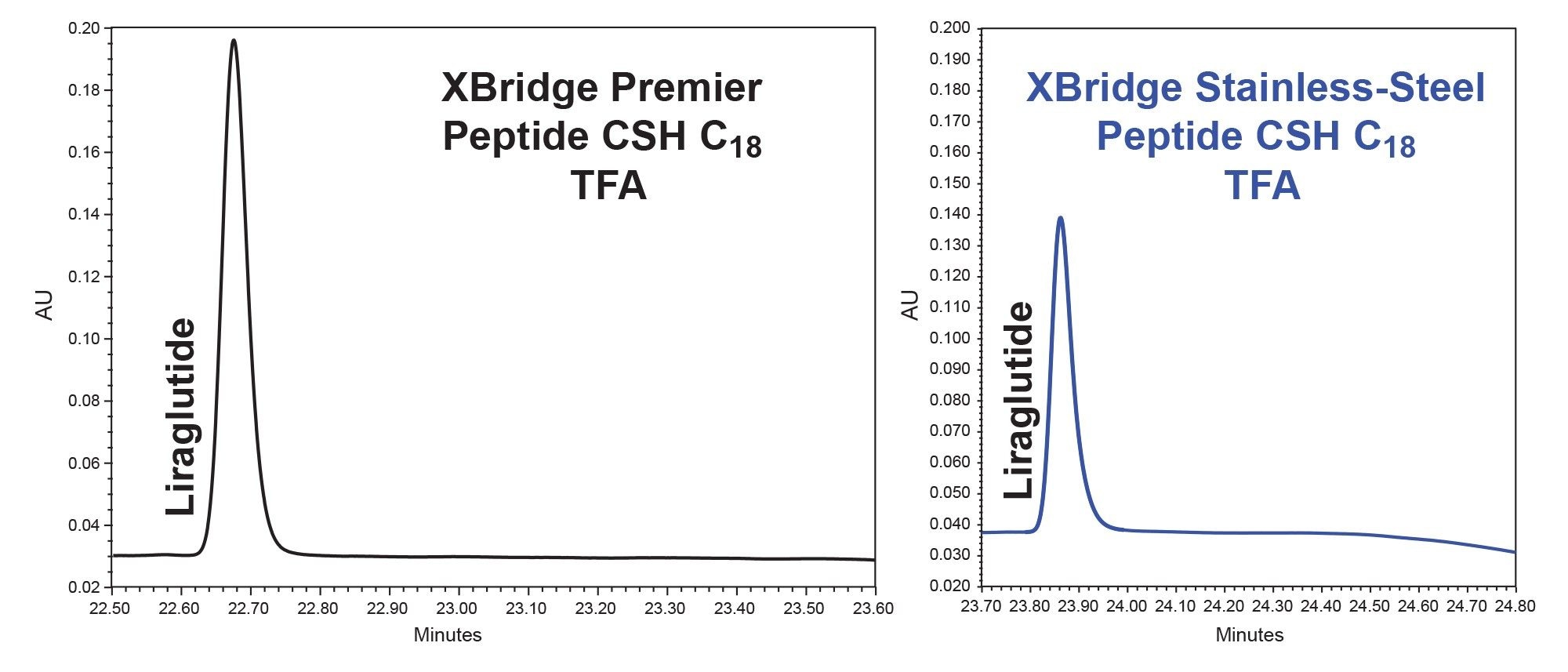
Size-exclusion chromatography (SEC) is a powerful technique for important size-based analyses of GLP-1 receptor agonists. MaxPeak Premier with High-Performance Surfaces (HPS) Technology reduces secondary interactions in SEC for accurate determination of high molecular weight products (HMWPs) and reproducible, high-resolution separations. MaxPeak Premier SEC Guards extend column life without sacrificing desired resolution and performance.
Scale the optimized RP analytical separation on Waters Peptide OBD Columns that can repeatedly deliver high-purity peptide product yields due to the outstanding stability of our RP particles packed in our OBD hardware. Achieve efficient lab scale peptide purifications with reduced costs.
Increase sensitivity for GLP-1 receptor agonists with MaxPeak Premier Peptide Columns, designed to minimize adsorption of acidic peptides (e.g., deamidated or phosphorylated) in optical and/or mass-based detection methods. Vanguard FIT Guards extend column life while maintaining peak shape, resolution, and efficiency. Available in multiple chemistries to accommodate different pH ranges to address your applications.
Achieve simple reversed-phase method development for peptides with Waters MaxPeak Premier Peptide Reversed-Phase Column Method Development Screening Kit. The kit includes a step-by-step guidance insert card to obtain a reasonable separation of 80% of your peptide sample mixtures in as few as 3 days. The two MaxPeak Premier Peptide Reversed-Phase Columns and standard were carefully selected to obtain desired component resolution by changing the separation selectivity.
Obtain fast, reliable extraction of GLP-1 RAs with Oasis HLB µElution Plates, designed to minimize peptide loss and support sensitive LC-MS quantitation.
Mitigate GLP-1 RA sample losses from non-specific binding (NSB) with QuanRecovery Vials and Plates. Designed for recovery, sensitivity, and reproducibility, these consumables minimize analyte-surface interactions with MaxPeak High Performance Surfaces (HPS) Technology.
Verify your column performance upon receipt and monitor column condition over column lifetime to save valuable research and development time with Waters MassPrep Peptide Standards.
Obtain orthogonal mass information on forced degradation impurities generated via oxidation, pH, and thermal stress with RapiZyme Trypsin, a rapid 30-minute enzymatic treatment.
Obtain accurate identification and quantification of amino acids with AccQ•Tag , a pre-column derivatization method and set of reagents for amino acid analysis (AAA) including hydrolyzed GLP-1 receptor agonists.
Streamline sample preparation for LC-MS analyses with verified workflows that help minimize variability, improve traceability, and simplify method transfer.
Explore how Waters service and support ensures your LC-UV/MS and LC-MALS are ready to deliver maximum performance and reliability when characterizing and testing GLP-1 RA pharmaceuticals, while Waters compliance services help you meet your GxP compliance needs.
Maximize your lab resources and minimize risk with payment options from Waters Capital, which includes innovative solutions to upgrade aging instruments, customized support, and flexible options to bundle your complete laboratory solution in one easy monthly payment – everything you need to advance your science.
Identify impurities of GLP-1 RAs with LC-UV/MS. Example analysis of Exenatide shown with high purity and MS spectra of the main species peak.
UV chromatograms of liraglutide drug substance and generic drug product showing the native hexamer and aggregate species. The molar mass measured by MALS is overlaid on each peak.

Developing a scalable separation method to obtain a high purity sample of GLP-1 RAs.
Mitigation of hydrophobic NSB to collection vessels using QuanRecovery with MaxPeak HPS 96-well Sample Collection plate, demonstrating improved MS peak response vs standard polypropylene plates for semaglutide.
