Method Migration of a Reversed Phase TFA-Acetonitrile Gradient Method From a Binary to a Quaternary System: Impact of Mixing
Abstract
Solvent mixing is critical to obtaining optimal gradient LC separations. Both high pressure and low pressure HPLC pump designs are subject to mobile phase composition fluctuations. Most modern HPLC Systems typically include a mixer in the pump design to reduce baseline noise and oscillations in mobile phase composition. In this study, the impact of mixers on chromatographic performance was demonstrated through the migration of a low wavelength, reversed phase TFA-Acetonitrile gradient method from a comparable binary HPLC System to the quaternary Alliance™ iS HPLC System. These method conditions are known to produce significant baseline noise (ripple) which can affect peak integration, sensitivity, and retention time precision. On the Alliance iS HPLC System, the optional 690 µL Ti diffusion bonded mixer was shown to produce decreased baseline noise resulting in improved baselines, more reproducible peak integration, and increased sensitivity compared to that of the binary and the quaternary systems with standard mixers.
Benefits
- Migration of low wavelength trifluoroacetic acid-acetonitrile gradient methods to the Alliance iS HPLC System is facilitated with the 690 µL Ti diffusion bonded mixer
- The 690 µL Ti diffusion bonded mixer reduces the characteristic ripple associated with low wavelength trifluoroacetic acid-acetonitrile gradient methods resulting in increased sensitivity, improved baseline stability, and easier peak integration
Introduction
Solvent mixing is critical to obtaining optimal gradient LC separations. The most commonly available pump mixing designs for reversed phase gradient HPLC separations are high pressure (typically binary) and low pressure (quaternary) systems. High pressure systems use independent pumps to deliver different solvents. Mixing occurs at high pressures with solvent composition controlled by the flow rates of the pump heads. In low pressure systems, solvent composition is controlled by a gradient proportioning valve. Each solvent is delivered in packets (based on the gradient specified in the method) which are mixed as they go through the pump head. High pressure systems have reduced dwell volumes and generally have improved compositional accuracy (leading to better retention time precision) over low pressure systems. Both high- and low-pressure systems are subject to mobile phase composition fluctuations. Mixers of various volumes and designs are utilized to help minimize these composition fluctuations by reducing baseline noise and oscillations in mobile phase composition.
The use of certain mobile phase modifiers is known to impact chromatographic baselines. Trifluoroacetic acid (TFA) is a commonly used ion-pairing reagent used in combination with acetonitrile for many gradient reversed phase applications. TFA absorbs strongly at wavelengths below 250 nm. Additionally, TFA is slightly retained on reversed phase columns which results in fluctuations in TFA concentration as the acetonitrile gradient passes through the column. In combination, these factors result in significant baseline disturbances (ripples) when TFA-acetonitrile gradients are used at low wavelengths. These baseline ripples may make peak integration difficult and can impact the sensitivity along with peak area precision and retention time precision of the method.
Most modern HPLC systems typically include a standard mixer in the pump design. Additionally, different mixers may be available to improve mixing performance for specific applications. The effect that mixers have on chromatographic performance was demonstrated during the migration of a reversed phase TFA-acetonitrile gradient method from a binary system to the quaternary Alliance iS HPLC System.
Experimental
METHOD: USP method for organic impurities in tryptophan (procedure 1)
Sample Description
USP Tryptophan Related Compound B (p/n: 1700523) was obtained from the U.S. Pharmacopoeia (Rockville, MD). A standard containing 1.0 mg/L of USP Tryptophan Related Compound B in water was prepared as described for the system suitability solution in the USP method for organic impurities in tryptophan (procedure 1).
LC Conditions
|
LC systems: |
Alliance iS HPLC System Comparable Binary HPLC system (System X) |
|
Detection: |
UV Detector with 10 mm HPLC flow cell |
|
Wavelength: |
220 nm |
|
Sampling rate: |
5 points/sec |
|
Vials: |
TruView pH Control LCMS Certified Clear Glass, 12 x 32 mm, Screw Neck Vial, with Cap and preslit PTFE/Silicone Septum, 2 mL Volume, 100/pk (p/n: 186005666CV) |
|
Column: |
XBridge™ C18 Column, 5 µm 4.6 x 250 mm (p/n: 186003117) |
|
Column temperature: |
30 °C |
|
Sample temperature: |
15 °C |
|
Injection volume: |
20 µL |
|
Flow rate: |
1.0 mL/min |
|
Mobile phase A: |
0.1% TFA in Water |
|
Mobile phase B: |
0.1% TFA in 80:20 Acetonitrile: Water |
|
Sample manager wash: |
90:10 Water: Acetonitrile |
|
Sample manager purge: |
0.1% TFA in Water |
|
Mixers: |
Alliance iS Stainless Steel 675 µL Bead Mixer (p/n: 700011656) Alliance iS 690 µL Ti Diffusion Bonded Mixer (p/n: 205002590) |
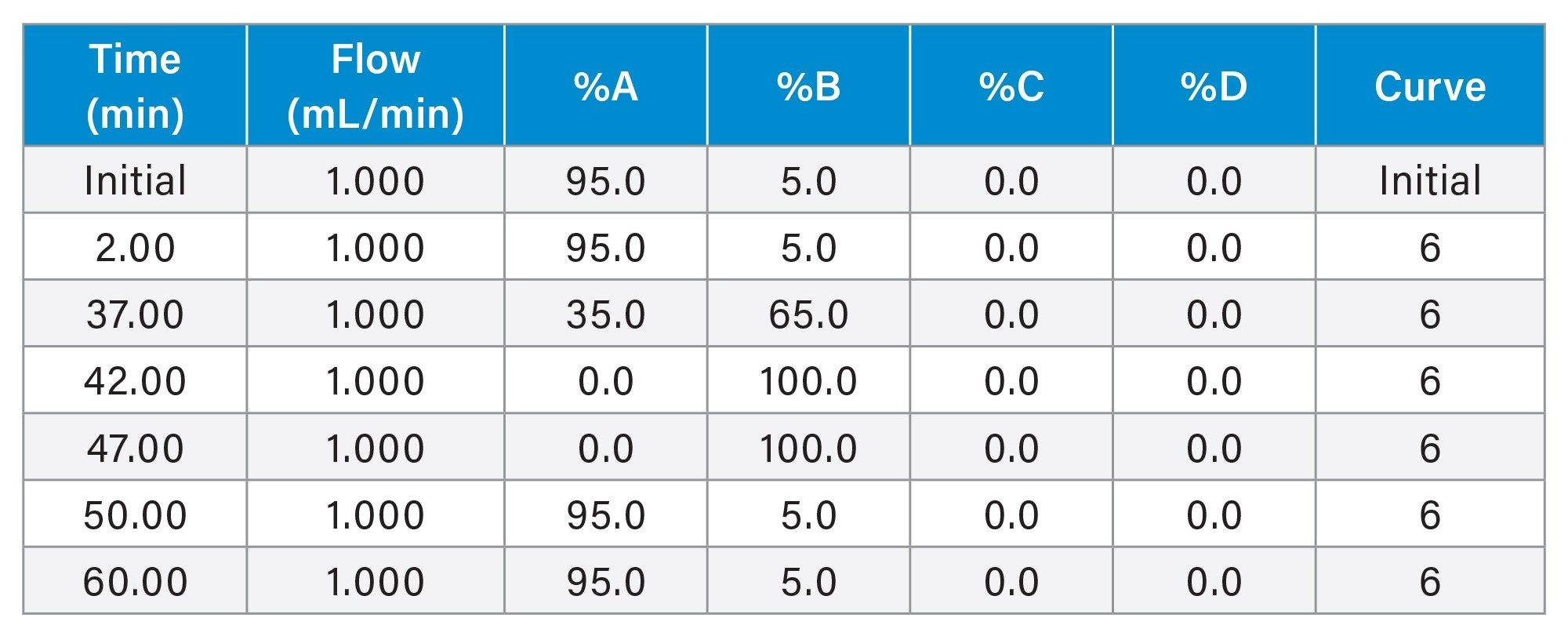
Gradient Table
Data Management
|
Chromatography data system: |
Empower™ 3.8.0.1 |
Results and Discussion
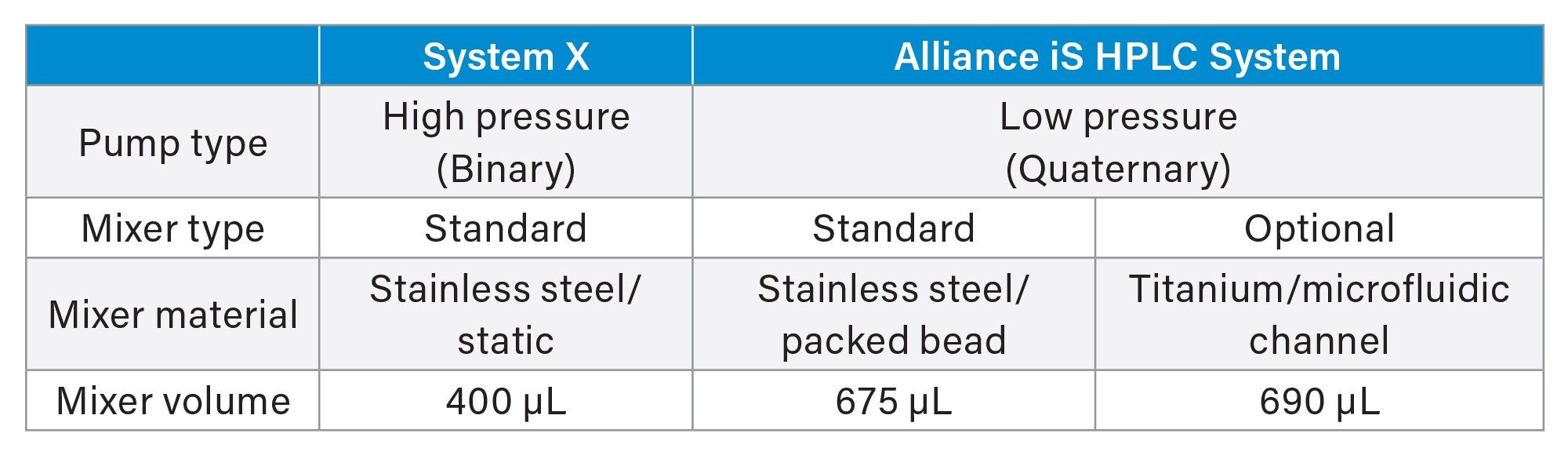
The impact of mixers on the migration of a reversed phase TFA-acetonitrile gradient method from a binary to a quaternary system was evaluated. The USP method for organic impurities in tryptophan (procedure 1) was migrated from a comparable binary system (System X) to the quaternary Alliance iS HPLC System. The method was run on System X (binary, originator system) with its standard 400 µL static mixer installed. Next, the method was migrated to the Alliance iS HPLC System (quaternary, receiver system) where it was evaluated with both the standard 675 µL stainless steel packed bead mixer and the optional titanium diffusion bonded mixer.
The titanium diffusion bonded mixer, developed for use with the Alliance iS HPLC System, has a microfluidic channel flow path that functions to blend solvents more efficiently than the standard packed bead mixer. This results in a reduction in baseline noise for select applications including TFA-Acetonitrile gradients at low wavelengths. The diffusion bonded mixer volume is nearly equivalent to that to that of the standard mixer (690 µL vs. 675 µL) therefore, significant delay volume is not added to the system when it is used. This is of particular importance in method migration as differences in delay volume can impact retention times.
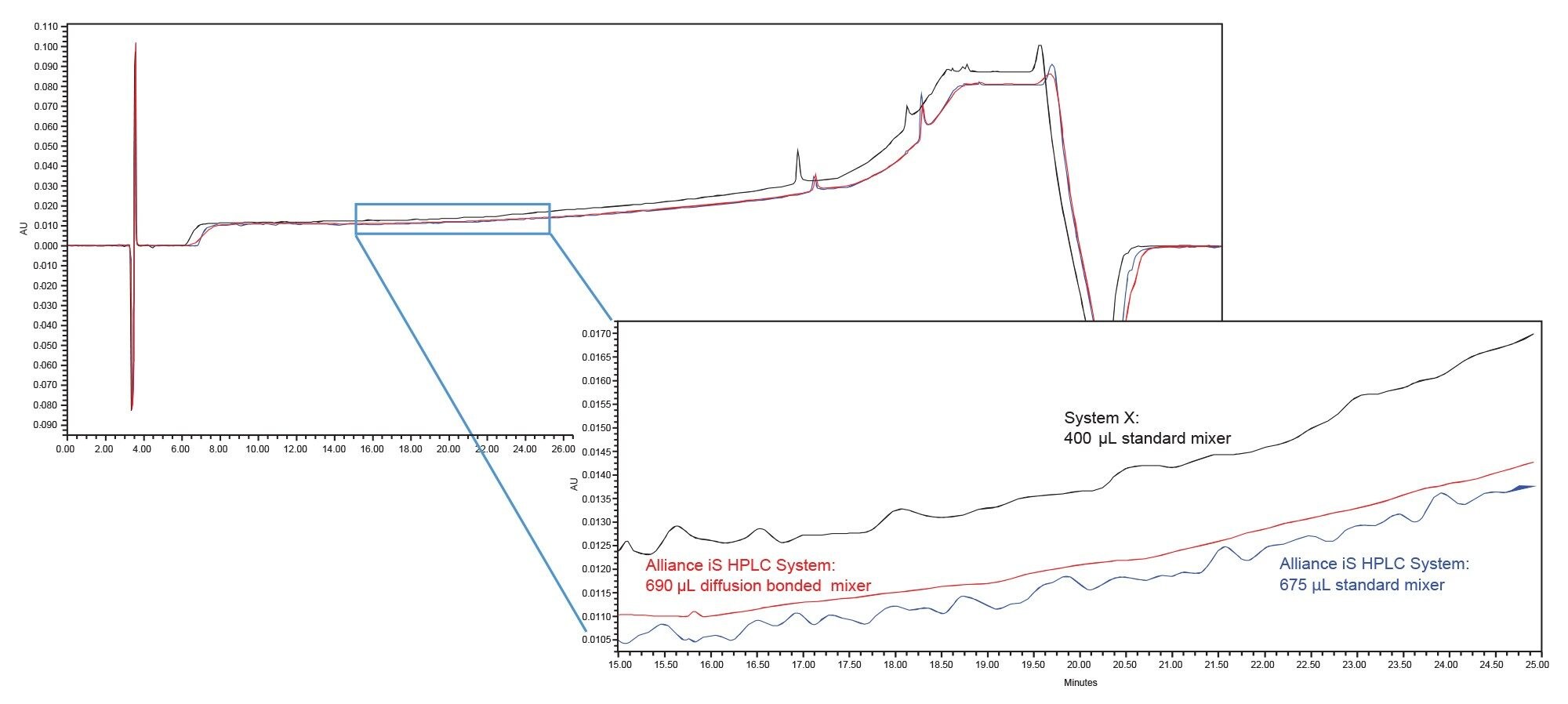
The impact of the different systems/mixers is displayed in Figures 1–4 which contain the chromatograms of both the blank (water) and system suitability solution injections. A visual comparison of the blank injections (Figure 1) shows the differences in baseline noise produced with each system/mixer. The effect of the 690 µL Ti diffusion bonded mixer is clearly seen with the baseline ripple reduced to less than that of the binary system.
Peak area precision, retention time precision and the USP signal to noise ratio for the tryptophan related compound B peak in the system suitability solution was calculated for each system/mixer. The USP Signal to noise ratio was used to define the sensitivity of each system. Figures 2–4 contain the chromatograms, and the results are summarized in Table 2.
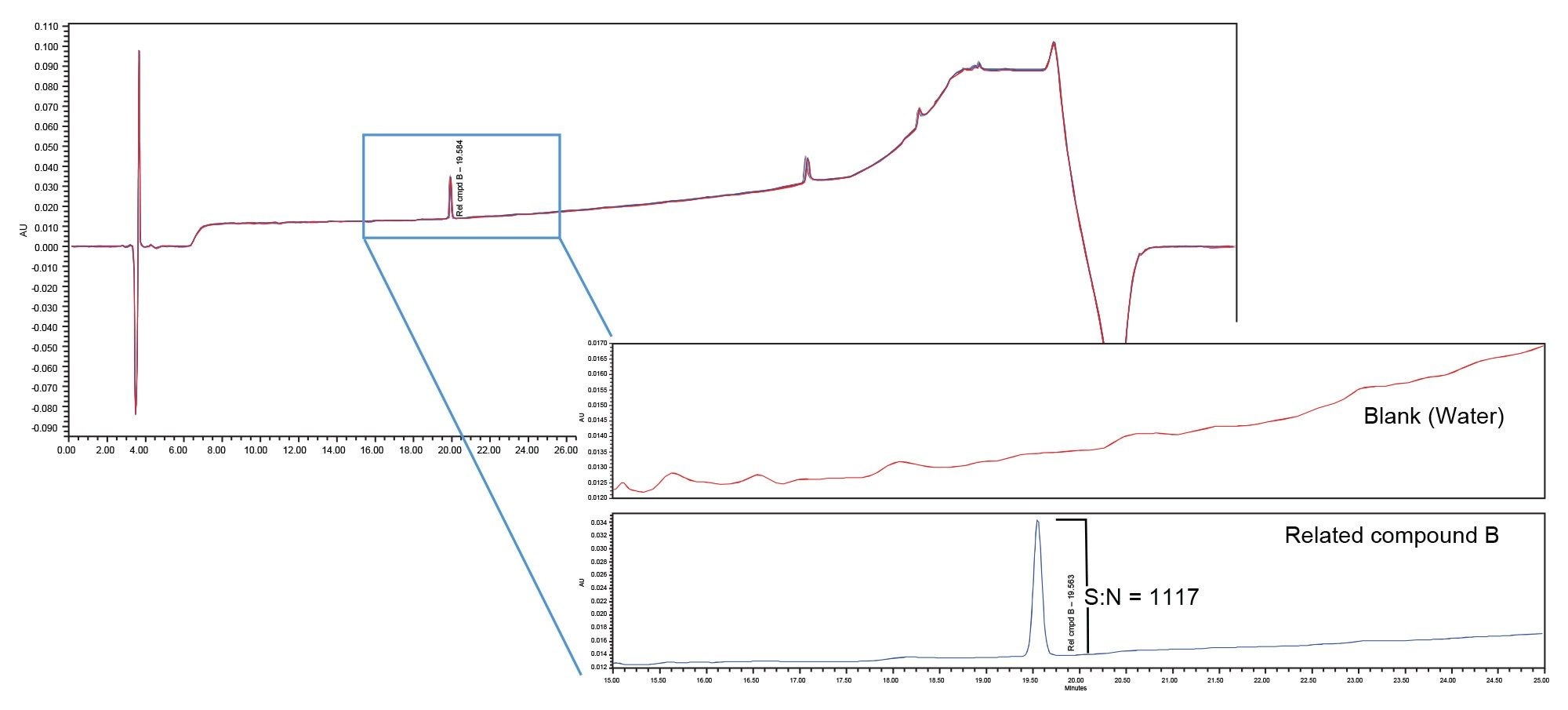
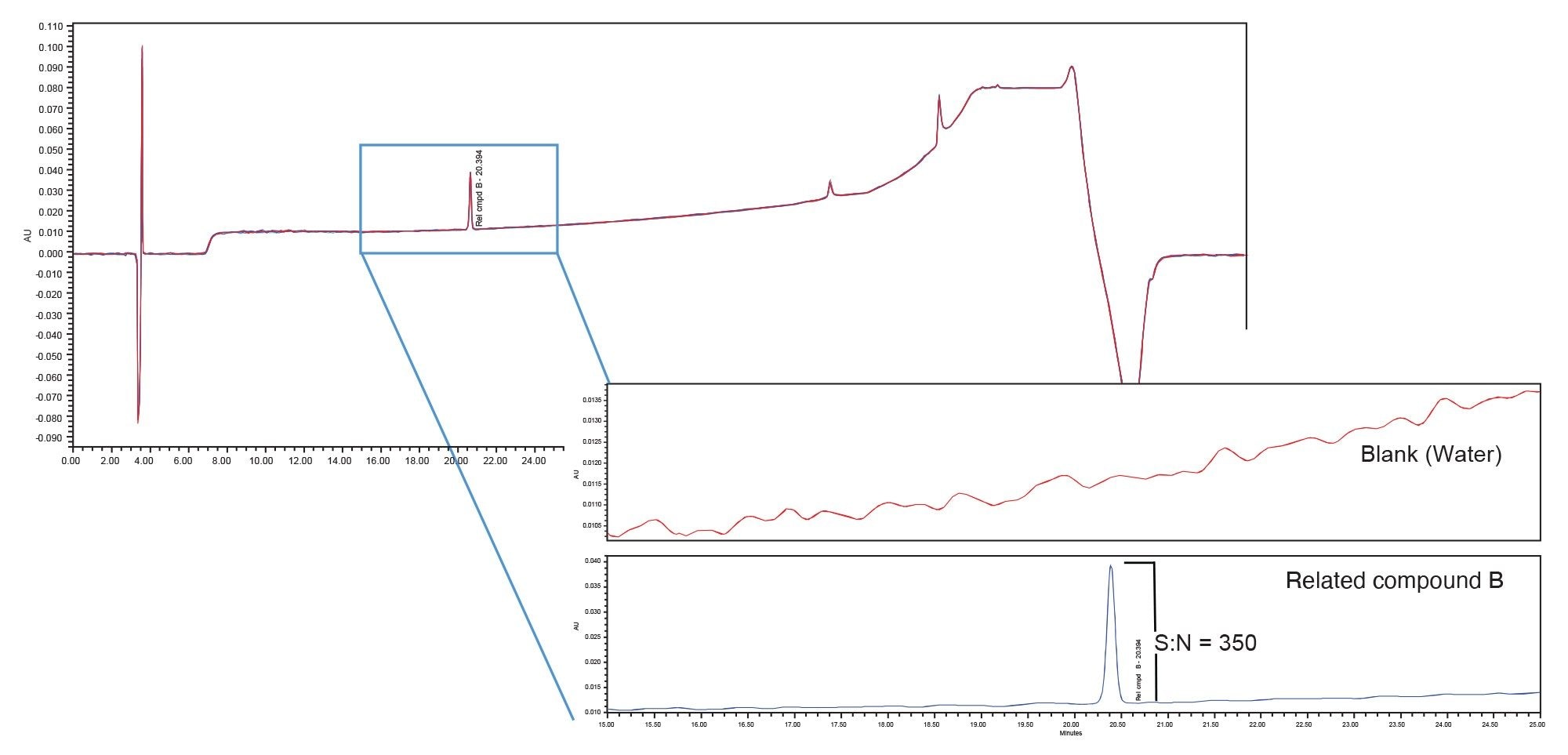
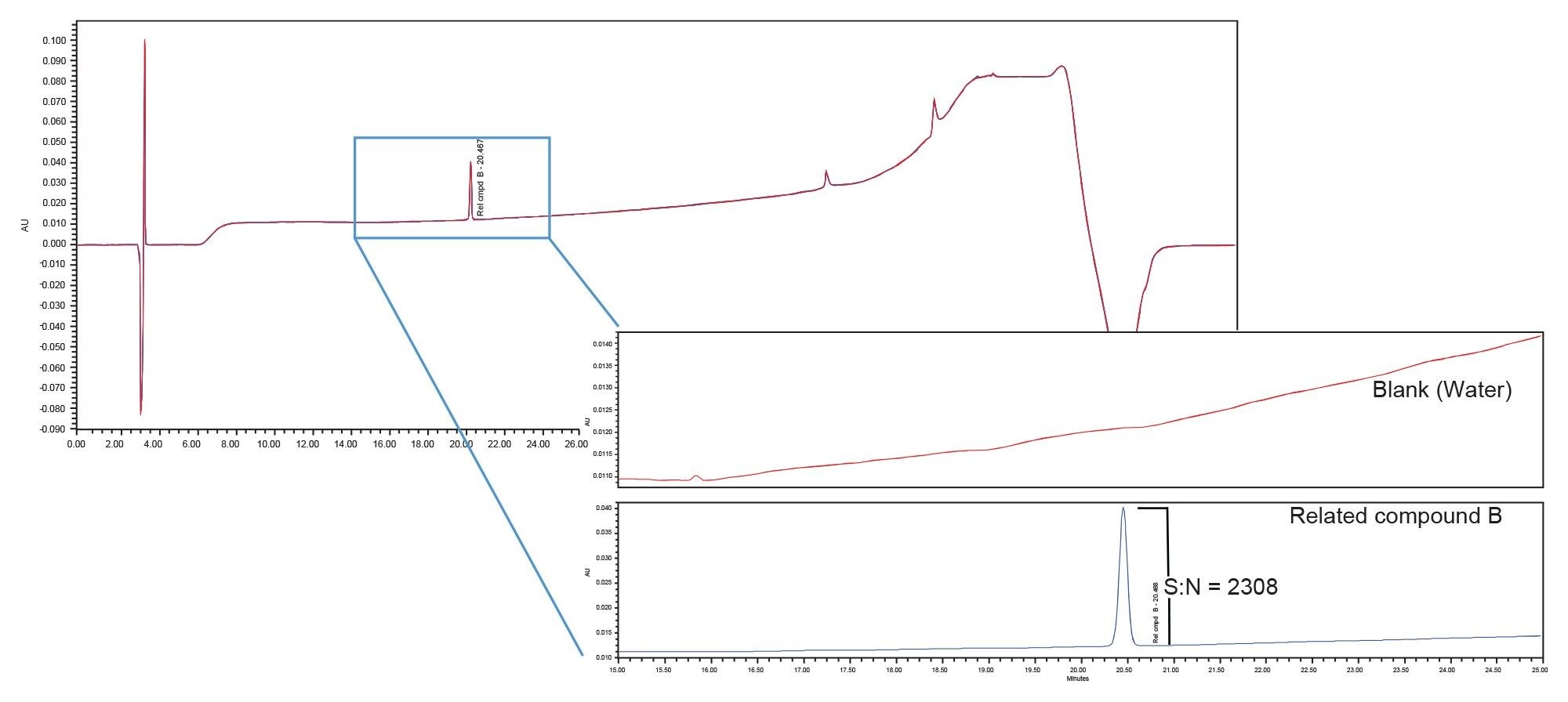
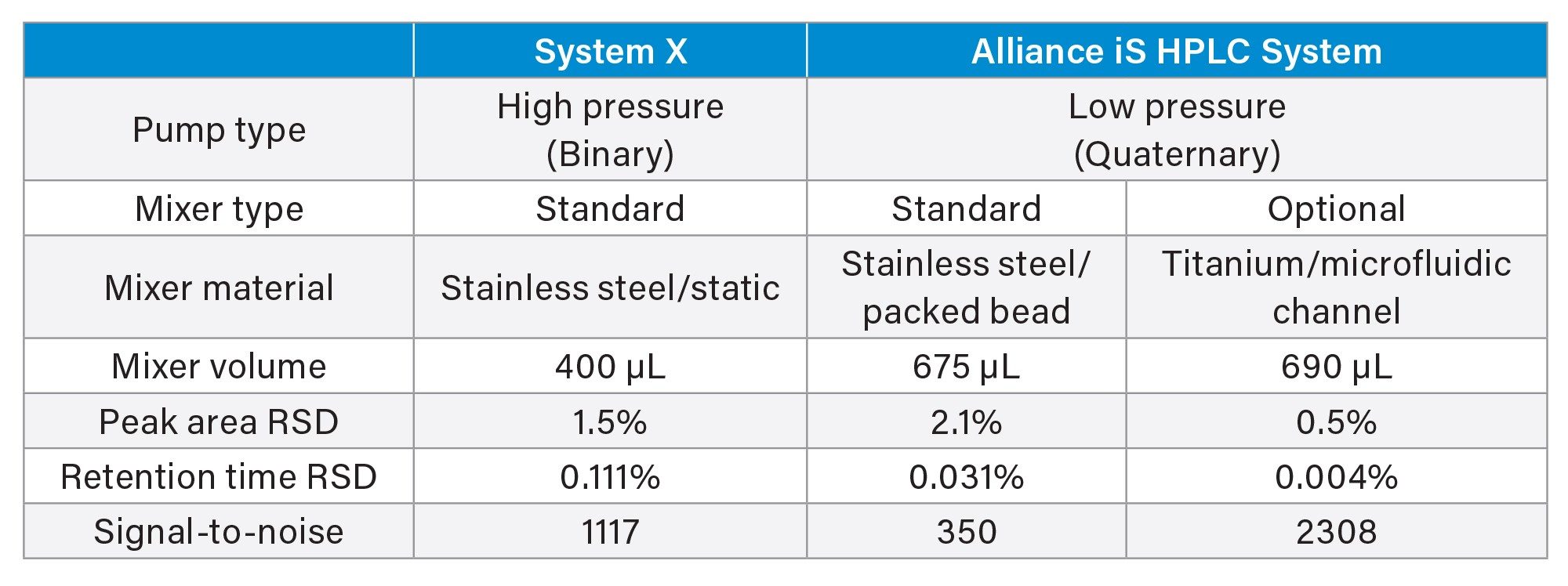
Sensitivity, defined by the USP signal to noise ratio of the tryptophan related compound B peak, was greatly impacted by the choice of mixer. The binary (originator) system with standard mixer produced signal to noise ratio of 1117. When the method was migrated to the Alliance iS HPLC System with its standard 675 µL mixer (receiver system) the signal to noise ratio was 350. This decrease in sensitivity from that of the binary system is a result of the increase in baseline noise (refer to Figure 1). When the 690 µL Ti diffusion bonded mixer was installed on the Alliance iS HPLC system, the signal to noise ratio increased to 2308, a 7x increase in sensitivity over that obtained with the standard 675 µL mixer and 2x increase over that of the binary system. This is due to a reduction in baseline noise and not because the peak signal was larger.
In addition to increasing sensitivity, improved baseline stability results in easier peak integration thereby reducing potential errors in peak identification and quantitation. Peak area and retention time reproducibility are also improved. The retention time and peak area reproducibility results for six replicate injections of the system suitability solution are presented in Table 2. There is good retention time precision across the systems/mixers, with the 690 µL Ti diffusion bonded mixer producing the lowest %RSD. The results for peak area precision also demonstrate the effect of improved baseline stability with 690 µL Ti diffusion bonded mixer, the system producing the smallest amount of baseline noise, yielding the smallest peak area %RSD.
Conclusion
The differences in mixing performance between binary and quaternary HPLC Systems may impact method migration. Mixing performance is particularly important when migrating gradient TFA-acetonitrile methods at low wavelengths, as these methods are known to produce significant baseline noise (ripple). In this study, effect of mixers on chromatographic performance was demonstrated through the migration of the USP Tryptophan Organic Impurities method from a binary system to the quaternary Alliance iS HPLC System. The results show that the characteristic baseline ripple associated with TFA-Acetonitrile gradients at low wavelengths is impacted by the choice of mixer. The Alliance iS HPLC System with the 690 µL Ti diffusion bonded mixer displayed a significant reduction of baseline noise when compared against both the binary and quaternary systems equipped with their respective standard mixers. This resulted in easier peak integration, improved peak area and retention time reproducibility, and increased method sensitivity.
References
- Jennifer Simeone, Paula Hong. Peptide Mapping using Binary Biocompatible LC Systems: Evaluation of Retention Time Precision and Mixing Effects on Waters and Competitive LCs. Waters Application Note. 720007078. March. 2021.
- United States Pharmacopeia (2023) USP Monographs Tryptophan Organic Impurities, USP-NF. DOI: https://doi.usp.org/USPNF/USPNF_M86750_05_01.html.
- Ti Diffusion Bonded Mixer – 690 µL Kit Installation Guide. User Manuals. 715009251. V00. Waters Corporation. June. 2024.
Featured Products
720008689, February 2025