Expanding the Antibody-Oligo Conjugate (AOC) Characterization Toolbox: Part 1- OAR Analysis and Conjugation Process Monitoring
Samantha Ippoliti, Ying Qing Yu, Connor Brandenburg, Tara MacCulloch, Michelle Chen
Waters Corporation, United States
Note: This is the first application note in a three-part series on AOC characterization. The second application note (720009066EN) detailed the intact AOC species characterization and served as an orthogonal analysis to already published SEC-MALS results (Wyatt White Paper WP8010). A third application note outlines a novel approach to conjugation site determination (720009079EN).
Published on October 24, 2025
Abstract
Antibody-oligonucleotide conjugates (AOCs) have emerged as a unique class of biotherapeutics that consists of an oligonucleotide chemically conjugated to a monoclonal antibody (mAb). Similar to antibody-drug conjugates (ADCs), the extent of conjugation relates to the efficacy and stability of the drug, and therefore, the conjugation must be fully characterized, followed by monitoring of key and critical attributes throughout drug development and commercialization. Many AOCs contain a double-stranded oligonucleotide component, such as an siRNA molecule, and therefore, typical denaturing LC-MS conditions are not feasible if one wishes to observe the fully formed product. This application note explores alternative non-denaturing LC-MS techniques such as SEC-MS and SCX-MS on the BioAccord™ LC-MS System for the characterization of AOC conjugation and oligonucleotide-to-antibody ratio (OAR), expanding detection capabilities to include high-resolution mass spectrometry.
Benefits
- Orthogonal non-denaturing techniques for AOC characterization, are all deployable on a benchtop time-of-flight (ToF) LC-MS system and accessible to users of all levels of MS-expertise
- Robust, reliable column chemistries and MS-grade IonHance™ mobile phase concentrates enable easy method setup and simplify transitions between assays
Introduction
AOCs are a class of biotherapeutics that are built upon the fundamentals and technology of ADCs. The purpose of both AOCs and ADCs is the targeted delivery of a payload (oligonucleotide moiety or cytotoxic drug, respectively) using a mAb to seek out and interact with a targeted cell population. These conjugated mAb therapeutics will likely provide the opportunity for new immuno therapies and cancer treatments.1,2 The AOC is comprised of the mAb, a linker (cleavable or non-cleavable), and the therapeutic oligonucleotide moiety (such as siRNA).2 The AOC used for this study consisted of an IgG1 isotype control mAb conjugated to double-stranded siRNA (siCOL1a1) via maleimidomethyl cyclohexane-1-carboxylate (MCC) linker (Figure 1).
The analysis of AOCs can be quite challenging. mAbs and oligonucleotides are large and complex, requiring extensive characterization and continued attribute monitoring during production. In addition to all the analytics applied to unconjugated mAbs and payloads, AOCs also require the determination of oligonucleotide-to-antibody ratio (OAR), which, like the drug-to-antibody-ratio (DAR) of an ADC, directly influences the efficacy and stability of the therapeutic. The OAR value, or the extent of conjugation, is measured throughout a conjugation reaction to identify the optimal process conditions and incubation times required to generate the desired product. Therefore, the analysis methods used to determine the OAR must be straightforward and have the ability for high-throughput analysis. For many ADCs, a typical reversed phase liquid chromatography coupled with mass spectrometry (RPLC-MS) or denaturing size-exclusion chromatography (SEC)-MS can often be employed to obtain DAR value. Many AOCs contain an oligonucleotide payload, such as double stranded siRNA, which cannot be analyzed via denaturing techniques. In the case of siRNA, the sense and antisense strands will become dissociated under these conditions. Therefore, non-denaturing chromatographic techniques are needed, such as SEC and ion exchange (IEX), that, when interfaced to MS, can determine the intact distribution and weighted average payload content (OAR) of the AOC species.
Many non-denaturing techniques require buffers and mobile phases that are not MS-compatible. However, advances in SEC and strong cation exchange (SCX) chromatography using volatile MS-grade ammonium acetate buffers help to bridge this gap.3 In this study, non-denaturing SEC-MS4 and SCX-MS were employed using the BioAccord LC-MS System for OAR analysis (Figure 1). This application note builds upon a previous AOC analysis5 conducted using SEC-MALS (multi-angle light scattering) and anion exchange (AEX)-MALS, to now incorporate high-resolution mass spectrometry into the toolbox for OAR determination and conjugation monitoring.

Experimental
Sample Preparation (free mAb and two AOCs)
The equivalent volume for 100 µg each sample was diluted to 1 mg/mL in 50 mM Ammonium Acetate, pH 6.8 (SEC-MS and SCX-MS). For mass check via RPLC-MS (free mAb only), the equivalent of 5 µg free mAb sample was diluted to 0.1 mg/mL in 0.1% formic acid in water. Samples were generously supplied by Takeda Pharmaceuticals.
Both AOC samples consisted of an IgG1 isotype control mAb conjugated to siCOL1a1 siRNA molecules via an MCC linker. Sample 1 had an average oligonucleotide to antibody ratio (OAR) of 1, and Sample 2 had an overall OAR of 2, as determined previously by SEC-MALS & AEX-MALS.5
LC Conditions
|
LC system (all): |
ACQUITY™ Premier System |
|
Detection (all): |
ACQUITY UPLC TUV Detector (280 nm) |
|
Sample temperature (all): |
6 °C |
|
Column(s): |
RPLC-MS: Waters™ ACQUITY Premier BEH™ C4 Column, 300 Å, 1.7 µm, 2.1 x 50 mm (p/n: 186010326) SEC-MS: ACQUITY Protein BEH SEC Column, 200 Å, 1.7 µm, 2.1 x 150 mm (p/n: 186008471) SCX-MS: BioResolve™ SCX mAb Column, 3 µm, 2.1 x 100 mm (p/n: 186009056) |
|
Column temperature: |
RPLC-MS: 80 °C SEC-MS & SCX-MS: 30 °C |
|
Injection volume: |
RPLC-MS: 10 µL 0.1 mg/mL free mAb (1 µg) SEC-MS & SCX-MS (intact): 10 µL 1 mg/mL AOC and free mAb (10 µg) |
|
Flow rate: |
RPLC-MS: 0.4 mL/min SEC-MS : 0.065 mL/min SCX-MS : 0.1 mL/min |
|
Mobile phases: RPLC-MS |
MPA: 0.1% Formic Acid in Water MPB: 0.1% Formic Acid in Acetonitrile |
|
Mobile phases: SEC-MS |
MPA (isocratic): 50 mM Ammonium Acetate, pH 6.8 (created from IonHance Ammonium Acetate Concentrate, p/n: 186009705) |
|
Mobile phases: SCX-MS |
MPA: IonHance CX-MS Concentrate A (p/n: 186009280), prepared as directed (10 mM Ammonium Acetate, pH 5.0) MPB: IonHance CX-MS Concentrate B (p/n: 186009281), prepared as directed (160 mM Ammonium Acetate, pH 8.5) |
Gradient Table: RPLC-MS
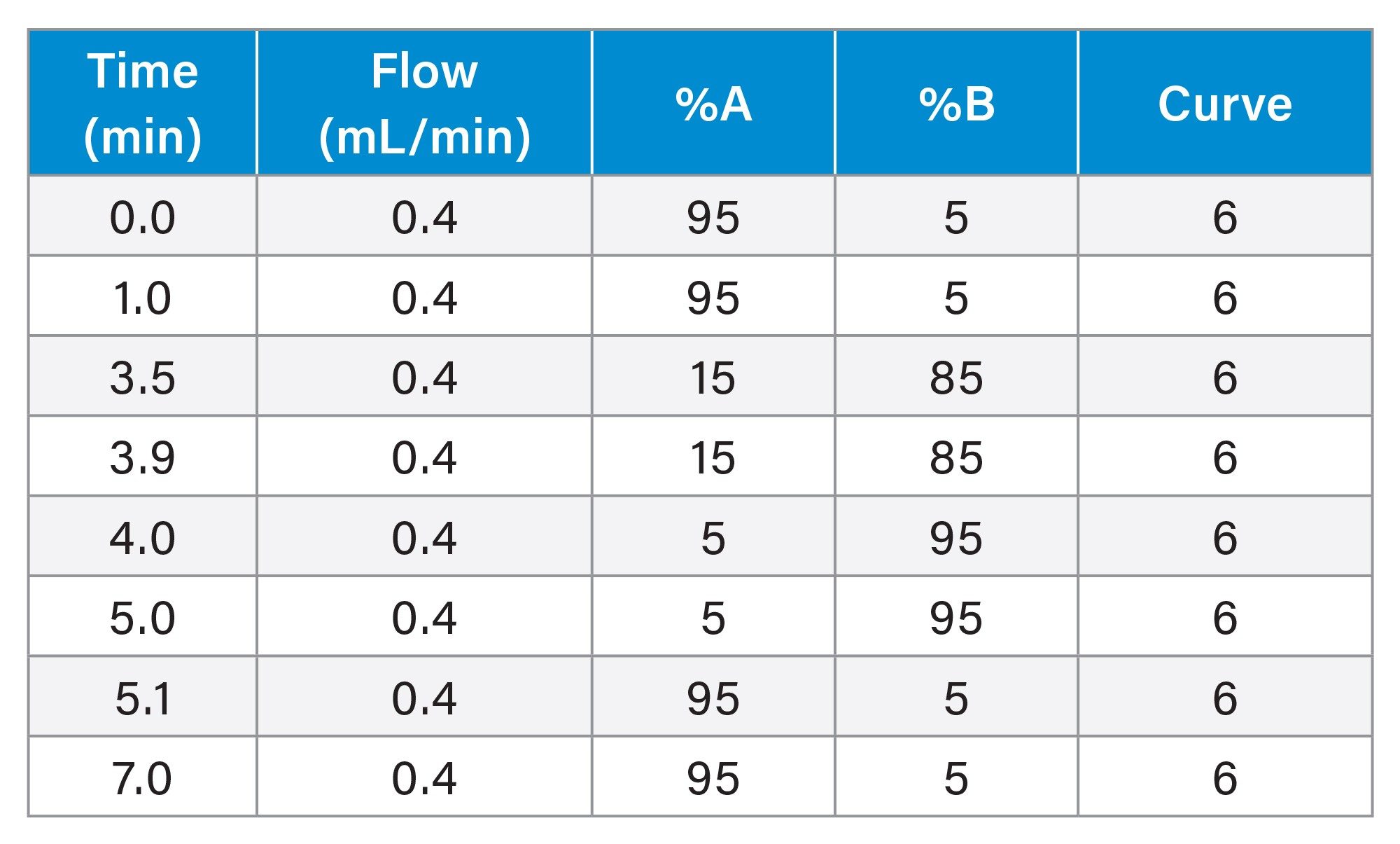
Gradient Table: SEC-MS
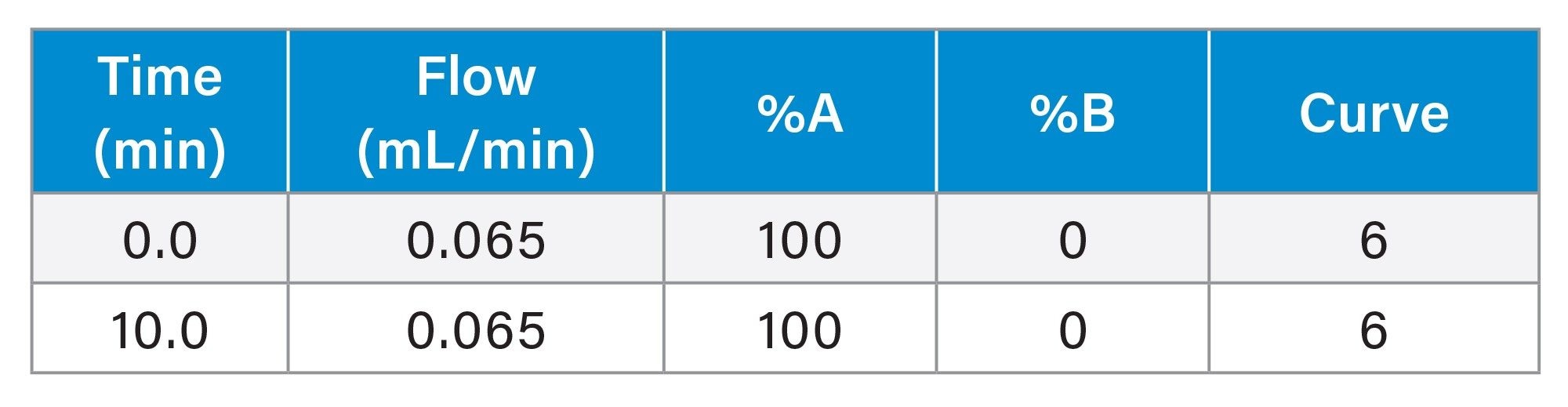
Gradient Table: SCX-MS (Intact mAb)
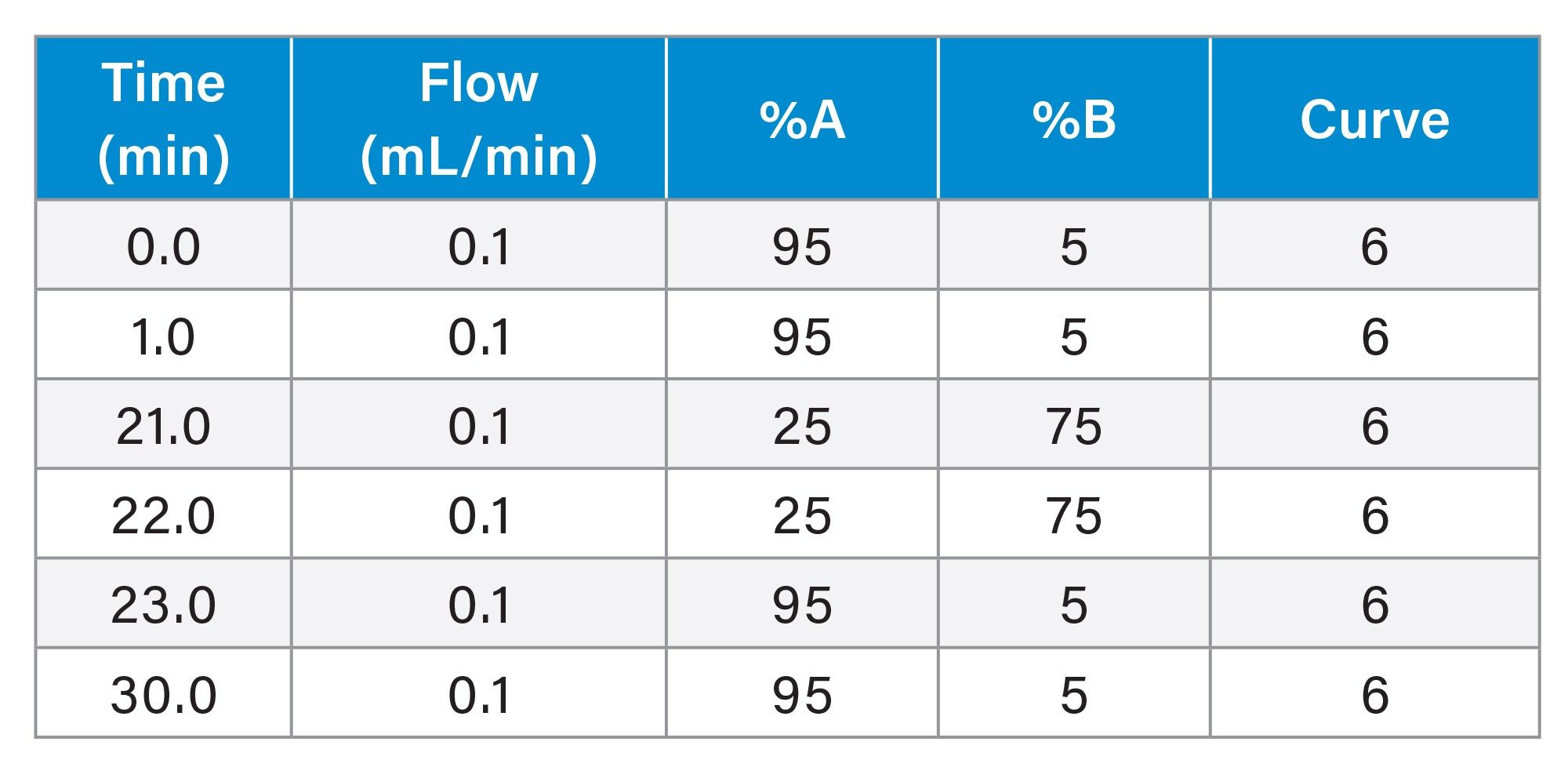
MS Conditions: RDa
|
MS system: |
ACQUITY RDa™ Detector |
|
Ionization mode: |
ESI Positive, Full Scan |
|
Acquisition range: |
400–7000 m/z (High Mass) |
|
Scan rate: |
2 Hz |
|
Capillary voltage: |
1.5 kV |
|
Cone voltage: |
RPLC-MS: 70 V SEC-MS and SCX-MS: 150 V |
|
Desolvation temperature: |
RPLC-MS: 550 °C SEC-MS and SCX-MS: 350 °C |
|
Lock mass: |
Standard |
Data Management
All data were acquired and processed through the UNIFI™ Application (v 2.1.2.4) and INTACT Mass App (v 1.9), all within the waters_connect™ Informatics Platform (v 3.1).
Results and Discussion
Establishing an accurate mass for the unconjugated free mAb can be performed by either RPLC-MS (denaturing) or SEC-MS (non-denaturing). The chromatographic separation in both RPLC-MS and SEC-MS (not shown) yielded a single main peak. The MS signal was summed across the main peak in each chromatogram and is displayed in Figure 2A (RPLC-MS in black, SEC-MS in red). The difference in charge state envelopes present in the denaturing (black) versus non-denaturing (red) analysis arose from the relative exposure of chargeable sites during the electrospray process. The non-denaturing analysis (which is necessary for intact AOC characterization) produced a range of lower charge states, found in the ~5000–7000 m/z range. Both the denaturing RPLC-MS and non-denaturing SEC-MS spectra provided deconvoluted masses that agreed with the theoretical mass (Figure 2B), including each mAb glycoform, which is not possible with light scattering detection techniques.
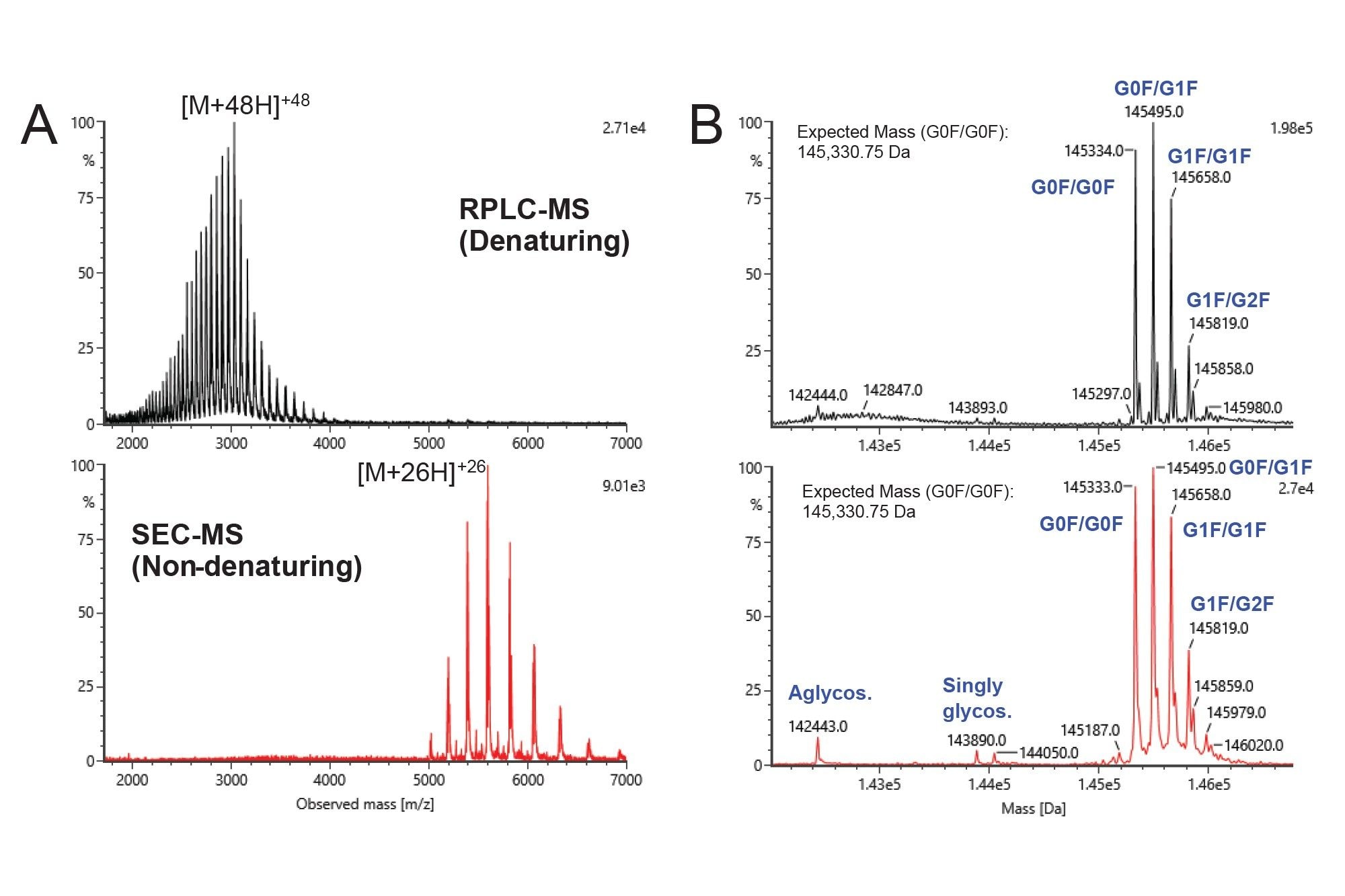
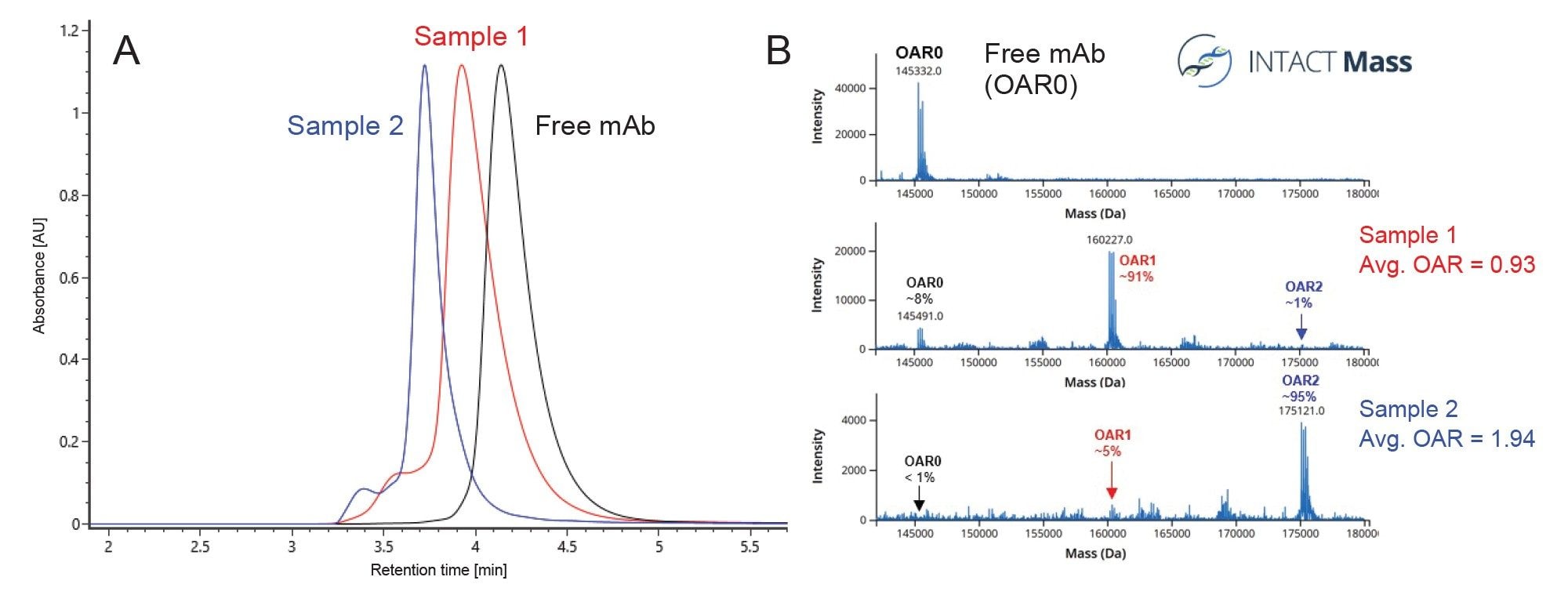
Once an expected mass for the unconjugated mAb has been established, SEC-MS analysis of the AOC samples can be performed (Figure 3). The UV overlay (Figure 3A) of the free mAb, Sample 1 (expected OAR of ~1.0), and Sample 2 (expected OAR of ~2.0), indicated a slight shift in hydrodynamic radius and/or molecular weight as the OAR value increases, as would be expected. For each analysis, the MS signal under the main peak was automatically combined, MaxEnt1-deconvoluted, and matched to expected masses within the INTACT Mass App. The deconvoluted spectra for each sample are shown in Figure 3B. SEC-MS confirmed the mass shifts for one and two conjugation sites occupied (~14.8 kDa and ~29.7 kDa, respectively), as expected for Sample 1 and 2. Small amounts of unconjugated and under conjugated mAb were also detected, which agrees with SEC-MALS and AEX-MALS results.5 The OAR values were automatically calculated by the weighted average of MS intensities of matched species within 50 ppm in the INTACT Mass App. (DAR calculation is available as of App version 1.9.) Sample 1 and Sample 2 were found to have experimentally calculated OAR values of 0.93 and 1.94, respectively.
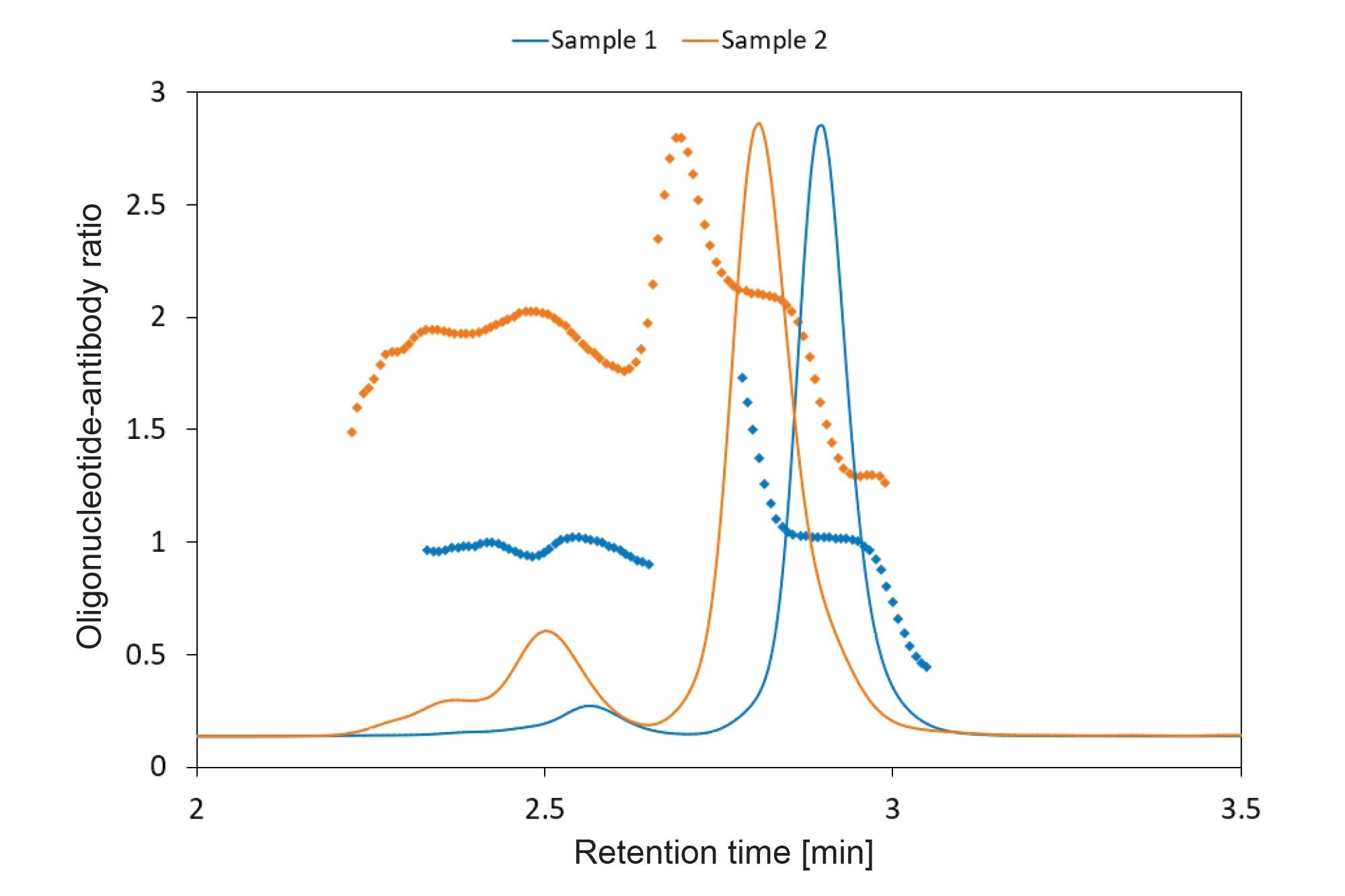
SEC-MS offers a very detailed characterization of the main monomer species present, but SEC-MALS can still provide critical orthogonal data. Namely, the SEC method used in this application note was adapted for MS compatibility and is, therefore, not fully equivalent to the method used for the referenced SEC-MALS5 analysis (Figure 4). The UV chromatogram overlay versus OAR value plots for Sample 1 (blue) and Sample 2 (orange) illustrate the utility of SEC-MALS to further assess size variants and determine OAR/molecular weight information of each species. The SEC-MALS chromatography method better maintains the integrity of the higher molecular weight (HMW) species for analysis and retains chromatographic resolving power for better quantification of aggregated species.
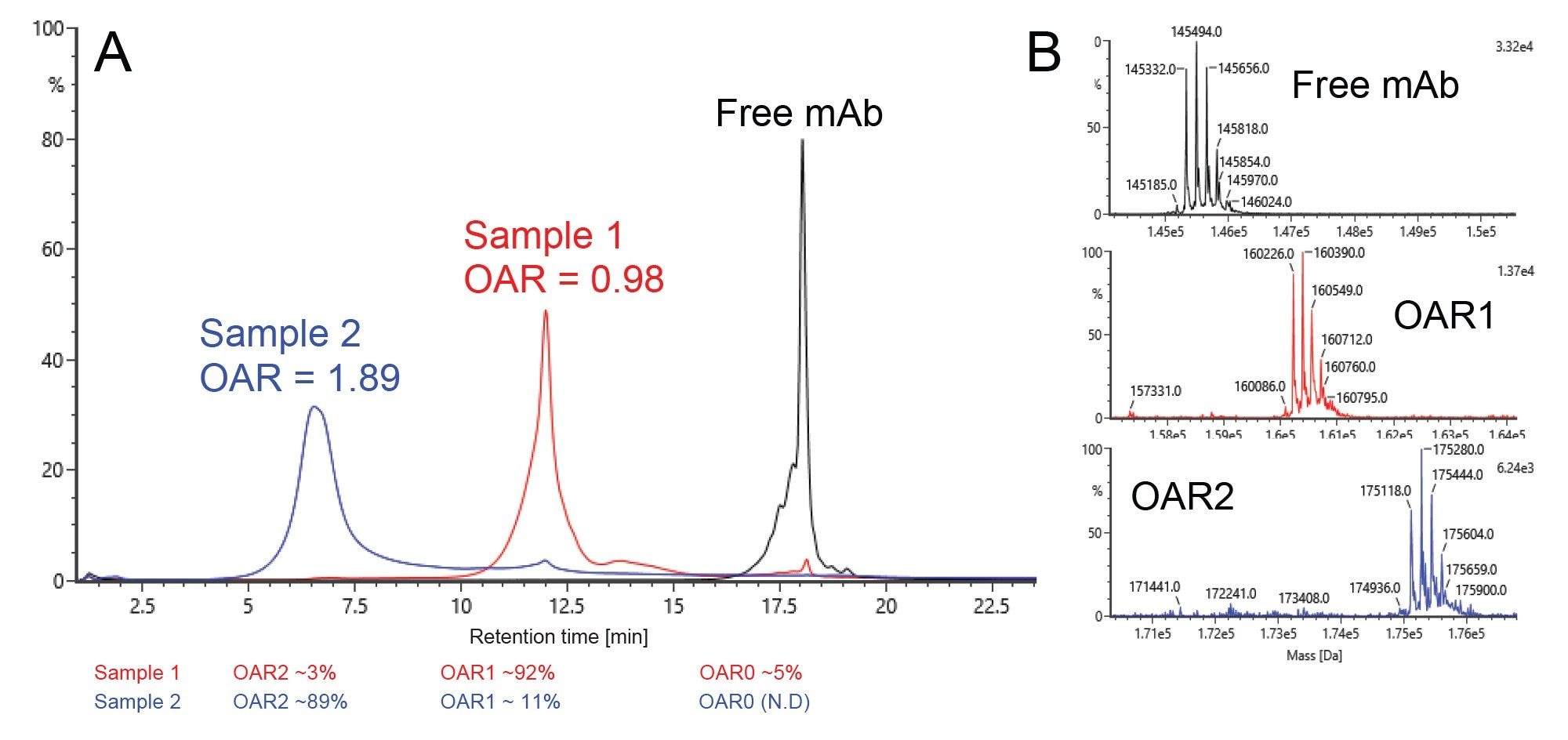
An alternative (also non-denaturing) LC-MS approach for OAR analysis is SCX-MS, which separates protein species by differences in surface charge. It has been predicted that this may be a useful tool in AOC analysis, as each time a negatively charged oligonucleotide moiety is added to the mAb, the overall surface charge will change. The overlay of UV chromatograms for SCX-MS analysis (Figure 5A) of the free mAb (black), Sample 1 (red), and Sample 2 (blue) generated results consistent with the data generated by SEC-MS. The resulting deconvoluted mass spectra for each of the main peak species (Figure 5B) also agreed with those obtained from SEC-MS. The use of a combined pH and salt gradient of MS-compatible mobile phases allows for more flexible chromatographic method optimization than SEC-MS (which uses simple isocratic flow and pore diffusion as the separation mechanism). Since baseline chromatographic resolution was possible via SCX-MS, the OAR can be calculated by integration of UV chromatogram or by integration of extracted ion chromatograms (XICs) for each OAR species.
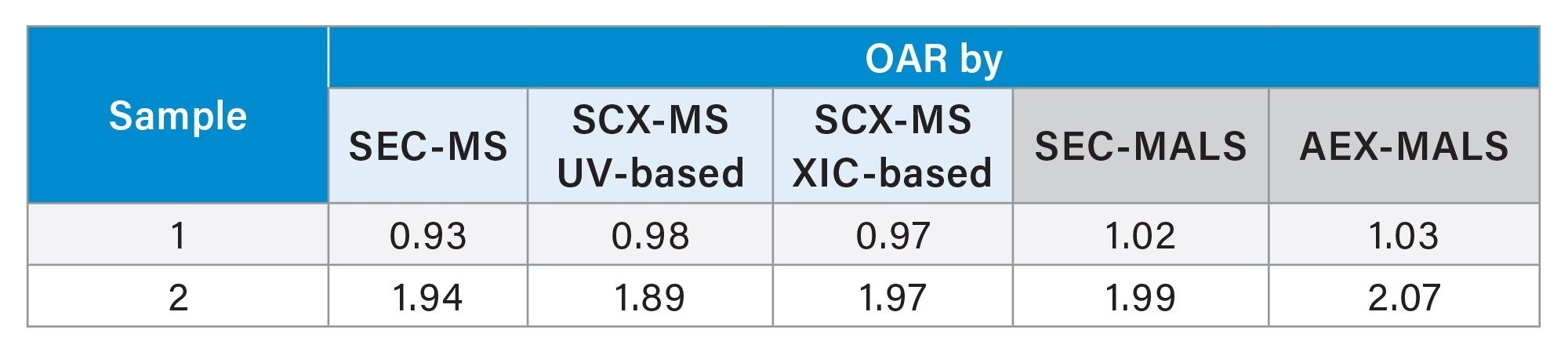
The OAR values determined by SEC-MS and SCX-MS were cross-referenced to previously reported SEC-MALS and AEX-MALS results5 (summarized in Table 1), showing excellent agreement of calculated OAR values across four orthogonal methods.
Conclusion
AOCs are an emerging biotherapeutic modality that requires analysts to reevaluate currently available characterization technologies used for the component parts to optimize methods for the combined molecule. This application note demonstrates the successful use of non-denaturing LC-MS assays employed on the BioAccord LC-MS System to characterize conjugation products and establish OAR values in support of AOC drug development. Both SEC-MS and SCX-MS techniques provided detailed and consistent mass spectral data supporting the design and optimization of a conjugation process and validating previous results obtained with orthogonal light scattering-based analytics.5 For OAR values and characterization of AOC monomer species present, high-resolution mass spectrometry detection (SEC-MS and SCX-MS) is preferred, while characterization of higher molecular weight species (such as aggregates) currently proves superior with light scattering techniques such as SEC-MALS and AEX-MALS.
References
- Dovgan I, Koniev O, Kolodych S, Wagner A. Antibody-Oligonucleotide Conjugates as Therapeutic, Imaging, and Detection Agents. Bioconjugate Chem. 30, 2483–2501 (2019). (http://doi.org/10.1021/acs.bioconjchem.9b00306)
- Jiao J, Qian Y, Lv Y, Wei W, Long Y, Guo X, Buerliesi A, Ye J, Han H, Li J, Zhu Y, Zhang W. Overcoming limitations and advancing the therapeutic potential of antibody-oligonucleotide conjugates (AOCs): Current status and future perspectives. Pharmacological Research. 209, 107469 (2024). (http://doi.org/10.1016/j.phrs.2024.107469)
- Ippoliti S, Schmudlach A, Lauber MA, Yu YQ. Online IEX-MS of mAb Charge Variants Using a BioResolve SCX mAb Column, IonHance CX-MS pH Concentrates, and BioAccord System. Waters Application Note. 720006672. 2019.
- Shion H, Doneanu C, Ha E, Yu YQ, Chen W. Analysis of Antibody siRNA Conjugate Using BioAccord System. Waters Application Note. 720007212. 2021.
- Brandenburg C, Liu H, Kenrick S. WP8010: Determination of Multiple Quality Attributes of Antibody-Oligonucleotide Conjugate with SEC-MALS and AEX-MALS. Waters-Wyatt White Paper. 2024.
Waters, BioAccord, IonHance, ACQUITY, BEH, BioResolve, UNIFI, waters_connect, and RDa are trademarks of Waters Technologies Corporation.
Featured Products
720009048, September 2025