Comprehensive Biotherapeutic Characterization Enabled by Electron Capture Dissociation on the SELECT SERIES™ Cyclic™ IMS Mass Spectrometer
Published on September 30, 2025
Abstract
During biotherapeutics development, the precise assessment of product quality attributes relies on peptide mapping using mass spectrometry. This approach allows for the identification and localization of protein modifications at both the peptide level and, with fragmentation capabilities, at the amino acid residue level. However, commonly available fragmentation techniques, such as collision-induced dissociation (CID), do not always yield the necessary information, potentially resulting in ambiguities. In this application note we describe the benefits of the electron capture dissociation (ECD) option on the SELECT SERIES Cyclic IMS mass spectrometer as a tool in peptide mapping workflows. ECD provides unique benefits over traditional dissociation techniques, which make it an attractive addition to the biopharmaceutical toolkit. We demonstrate the utility of ECD for the localization of glycosylation sites, for the distinction of aspartic and isoaspartic acid products of deamidation, and for the confirmation of leucine and isoleucine incorporation into the protein sequence. We also show the characterization of disulfide bonded peptides, something often intractable by collision-based approaches. Furthermore, the compatibility of the ECD implementation with ion mobility extends the possibilities concerning isomeric peptide characterization, which we show with Asp/isoAsp peptides.
Benefits
- Fully characterize protein-based therapeutics with the flexibility of fragmentation techniques on the SELECT SERIES Cyclic IMS
- Determine the sites of labile modifications such as glycosylations using ECD
- Unambiguously distinguish aspartic and isoaspartic isomers by combining ECD with high resolution ion mobility
- Confidently confirm the presence of leucine and isoleucine from tandem MS spectra using characteristic w ions
- Achieve comprehensive fragmentation of large peptides with ECD, including disulfide-bonded peptides
Introduction
The ability to fully characterize protein-based therapeutics during their development is central to analytical laboratories in pharmaceutical companies and contract research organizations. Peptide mapping by mass spectrometry is the gold standard approach to identifying and relatively quantifying critical quality attributes including post-translational modifications (PTMs) and degradation products. Important PTMs include glycosylations, which must be monitored and controlled due to their critical role in the stability, activity, and immunogenicity of biotherapeutic substances. Products of degradation include oxidation, deamidation and amino acid isomerization, which can also significantly affect product activity. The sites of modification are typically identified by tandem mass spectrometry utilizing the established fragmentation technique collision-induced dissociation (CID). CID, however, it is not suitable for all modification types. For example, the glycans present in glycopeptides will often fragment preferentially relative to the peptide backbone, which precludes identification of the glycosylation site. Moreover, certain isomeric amino acid pairs such as aspartic/isoaspartic acid and leucine/isoleucine produce identical product ion spectra meaning their presence cannot be confirmed de novo.
Electron capture dissociation (ECD) is an emerging fragmentation technique available as an option on the SELECT SERIES Cyclic IMS instrument.1,2 Like CID, ECD produces sequence-confirming ions but of a different type; CID produces b and y-type ions whereas ECD yields c and z-type ions. Due to its radical-mediated mechanism, ECD has the benefit of providing complementary information to CID and has unique capabilities with certain PTMs, degradation products, and isomeric amino acids. For glycopeptides ECD occurs predominantly on the peptide backbone which allows determination of the glycan attachment site. For deamidations, which often produce isomeric aspartic acid and isoaspartic acid products, ECD can produce characteristic product ions that allow their distinction.3 Spontaneous aspartic acid isomerization, i.e. without prior deamidation of an asparagine, can also be distinguished in the same way. Furthermore, ECD produces characteristic product ions for isomeric leucine and isoleucine in peptides containing these residues, facilitating confirmation of each directly from tandem MS spectra.4 An additional assay conducted at the peptide level is disulfide mapping, used to confirm intra- and interchain crosslinks that have a central role in protein conformational stability. Unlike CID, ECD has the ability to cleave disulfides and therefore the potential to increase the information gained in these assays.5
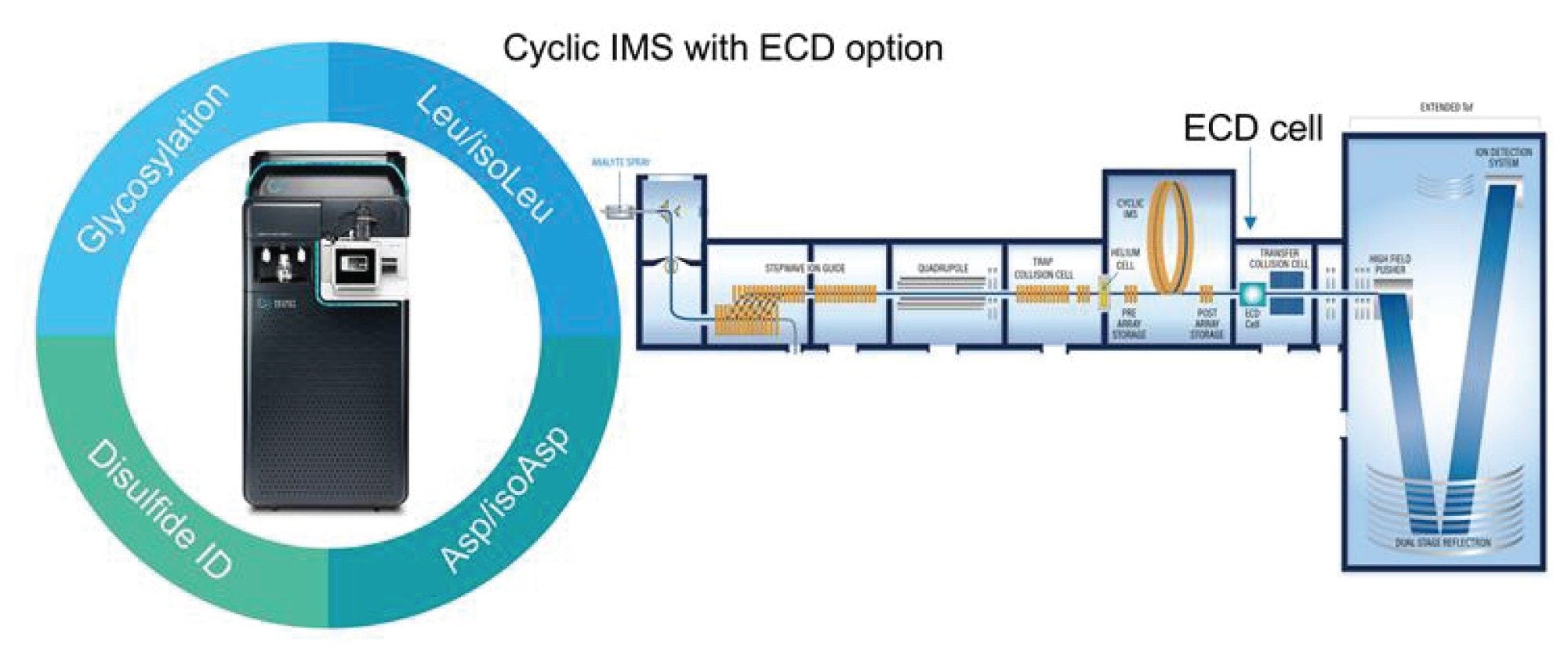
Experimental
Sample Description
Waters™ mAb tryptic digestion standard (NISTmAb RM8671, Waters p/n: 186009126) reconstituted to a final concentration of 1.3 µM in mobile phase A
LC Conditions
|
LC system: |
ACQUITY™ Premier UPLC™ |
|
Vials: |
Total recovery vials (p/n: 186002805) |
|
Column(s): |
ACQUITY Premier Peptide CSH™ C18, 130 Å, 1.7 µm, 2.1 × 100 mm (p/n: 186009488) |
|
Column temperature: |
45 °C |
|
Sample temperature: |
8 °C |
|
Injection volume: |
2 µL |
|
Flow rate: |
0.3 ml/min |
|
Mobile phase A: |
Water +0.1% formic acid |
|
Mobile phase B: |
Acetonitrile +0.1% formic acid |
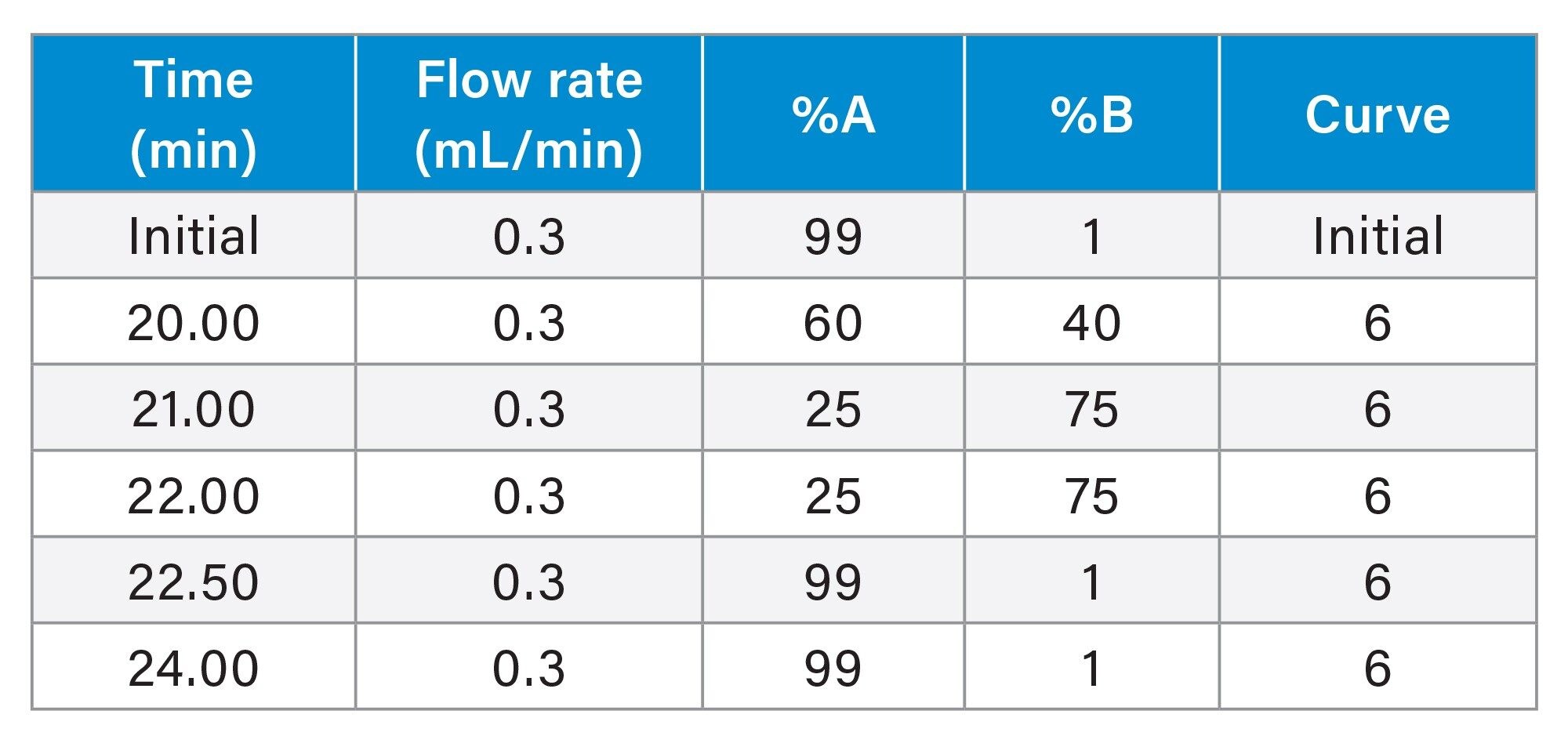
Gradient Table
MS Conditions
|
MS System: |
SELECT SERIES Cyclic IMS |
|
Polarity: |
Positive |
|
Acquisition range: |
100–2,000 or 4,000 m/z |
|
Capillary voltage: |
2 kV |
|
Acquisition mode: |
TOF DDA / HDMSMS |
|
Scan time: |
0.3 s |
|
Fragmentation mode: |
ECD |
|
Cone voltage: |
40 V |
|
Desolvation temperature: |
550 °C |
|
Desolvation gas flow: |
800 L/hr |
|
Source temperature: |
120 °C |
|
Cone gas: |
0 |
|
Nebulizer: |
6 |
|
ECD cell: |
Waters ECD cell option |
Data Management
|
MS acquisition software: |
Masslynx™ 4.2 SCN 1016 Version 9 |
|
Data interpretation software: |
waters_connect™ UNIFI™ biopharma applications |
Results and Discussion
Localization of Glycosylation Sites
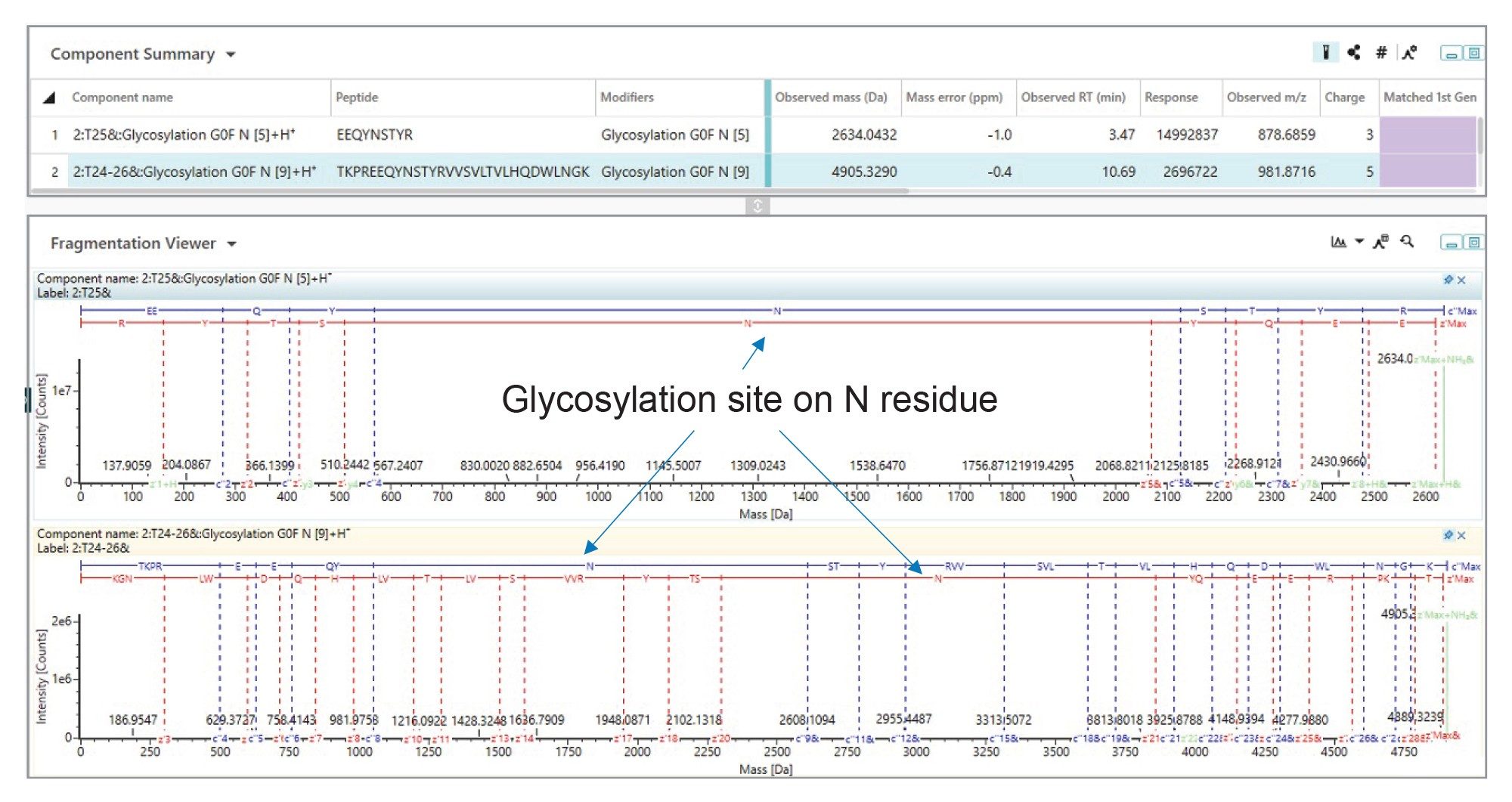
To confirm the glycosylation site of the major N-glycan from NISTmAb the peptides T25 and the miscleaved T24-26 from the heavy chain were subjected to ECD fragmentation (TOF DDA with an inclusion list). Figure 2 shows an extensive series of c and z product ions, which are formed during ECD, enabling full backbone coverage for both peptides and confirmation of the glycosite as asparagine N300 from the heavy chain.
Distinguishing Aspartic acid and Isoaspartic Acid
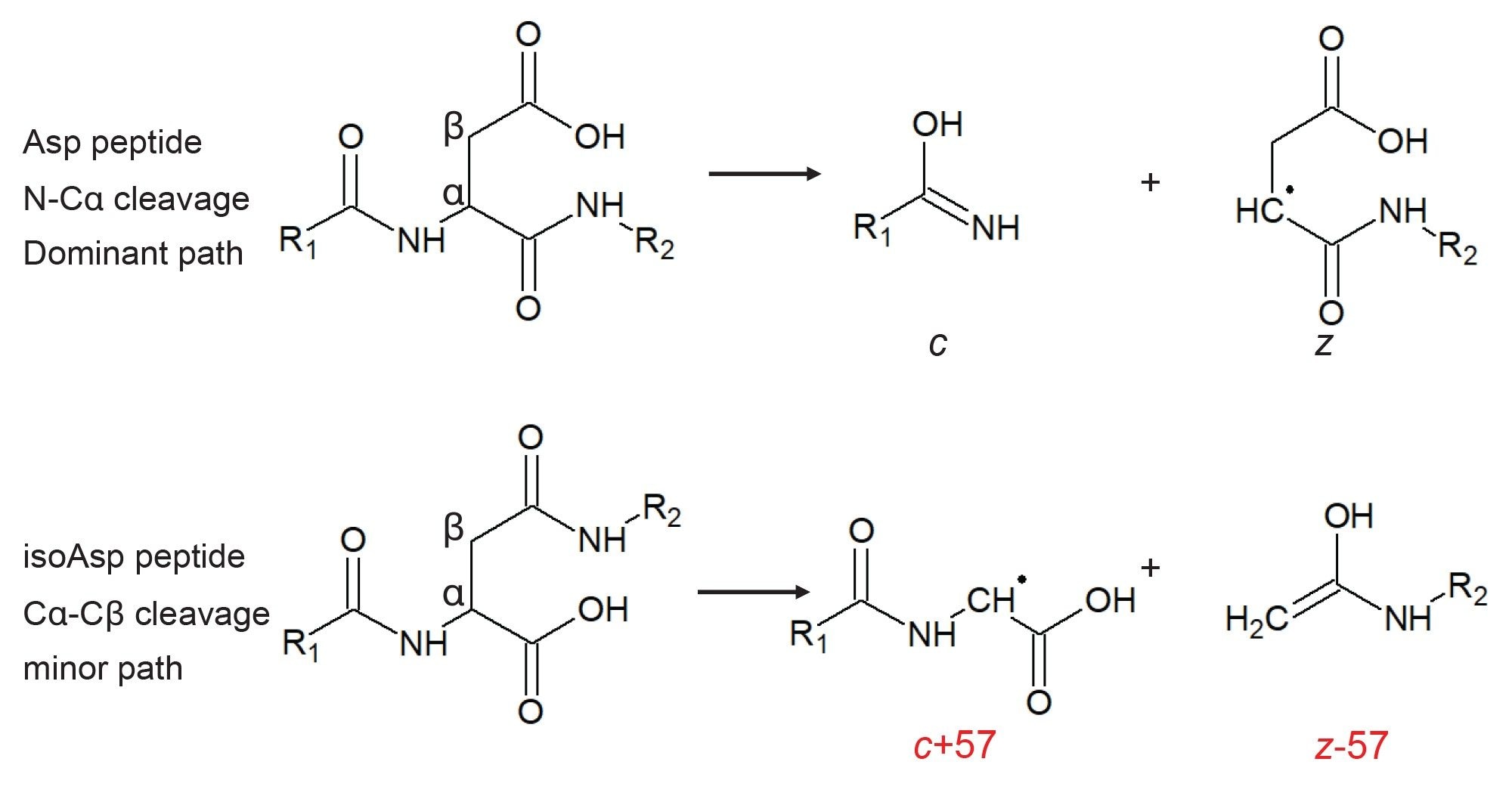
Isoaspartic acid can form both spontaneously at aspartic acid residues and as a result of isomerization after the deamidation of an asparagine. In both cases, the detected isoAsp-containing peptide would have an identical mass to the Asp form, eventually leading to two distinct peaks in the chromatogram. In an isoAsp-containing peptide, the presence of either z-57 Da or c+57 Da signals can indicate its presence. For example, in a 10 amino acid peptide with isoAsp at position 5 the z6-57 and c4+57 ions are possible. Detection of either ion, or both, would confirm the isoAsp residue at that position. In our peptide mapping results from NISTmAb, we detected two minor chromatographic peaks eluting at retention times (at 12.75 and 12.87 mins) later than the peptide T26 from the heavy chain (12.53 minutes) with masses 0.98 Da higher (Figure 4). This observation suggests deamidation, and the fact that we detect two signals indicates Asp isomerism. Indeed, inspection of the ECD-DDA data using the peptide mapping tools in waters_connect revealed product ions at 246.14 m/z and 1564 m/z corresponding to z3-57 and c13+57, respectively, originating from the species at 12.75 min, indicating that this is the isoAsp peptide. The absence of these signals from the peak at 12.87 minute confirms this assignment.
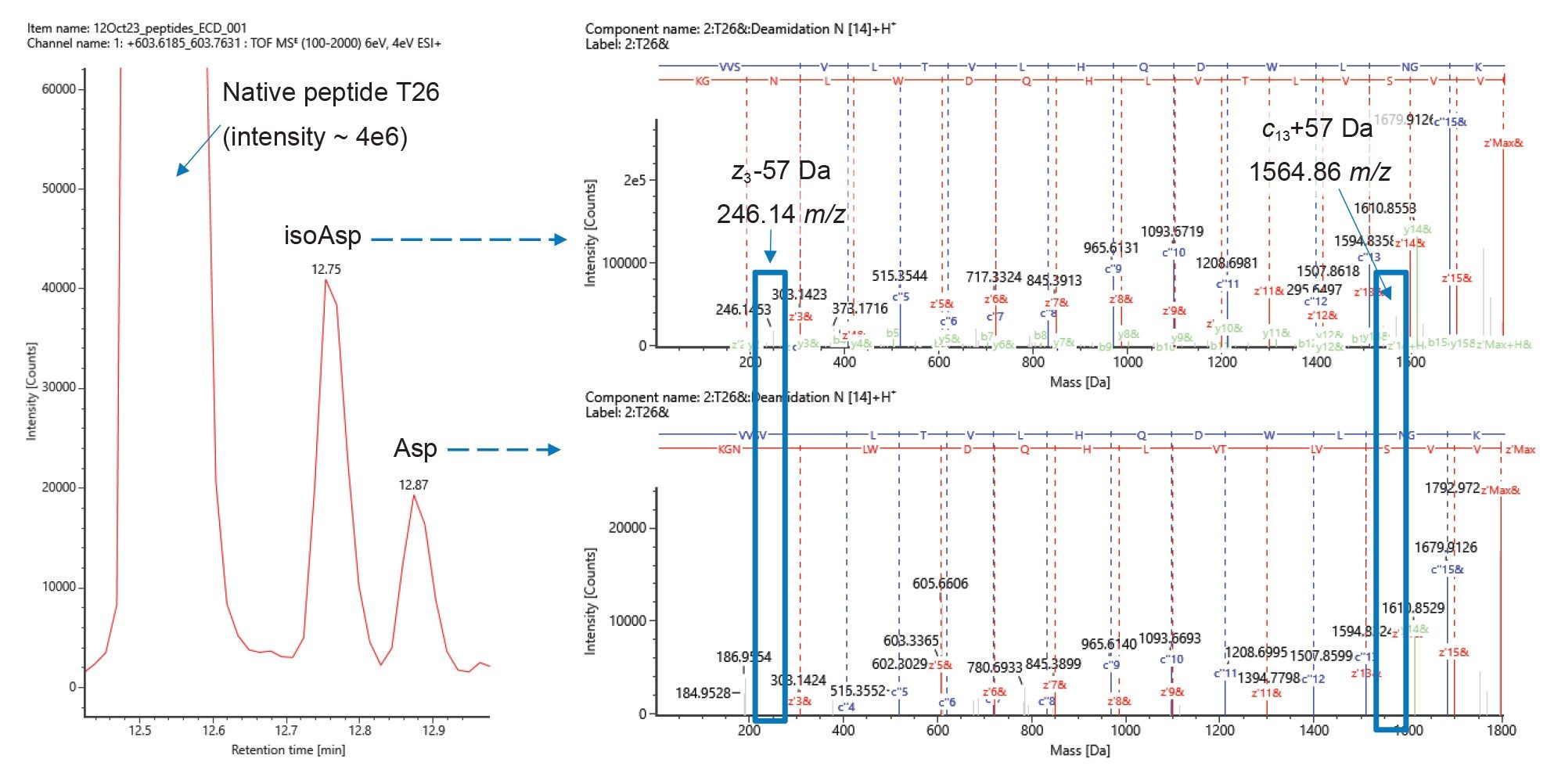
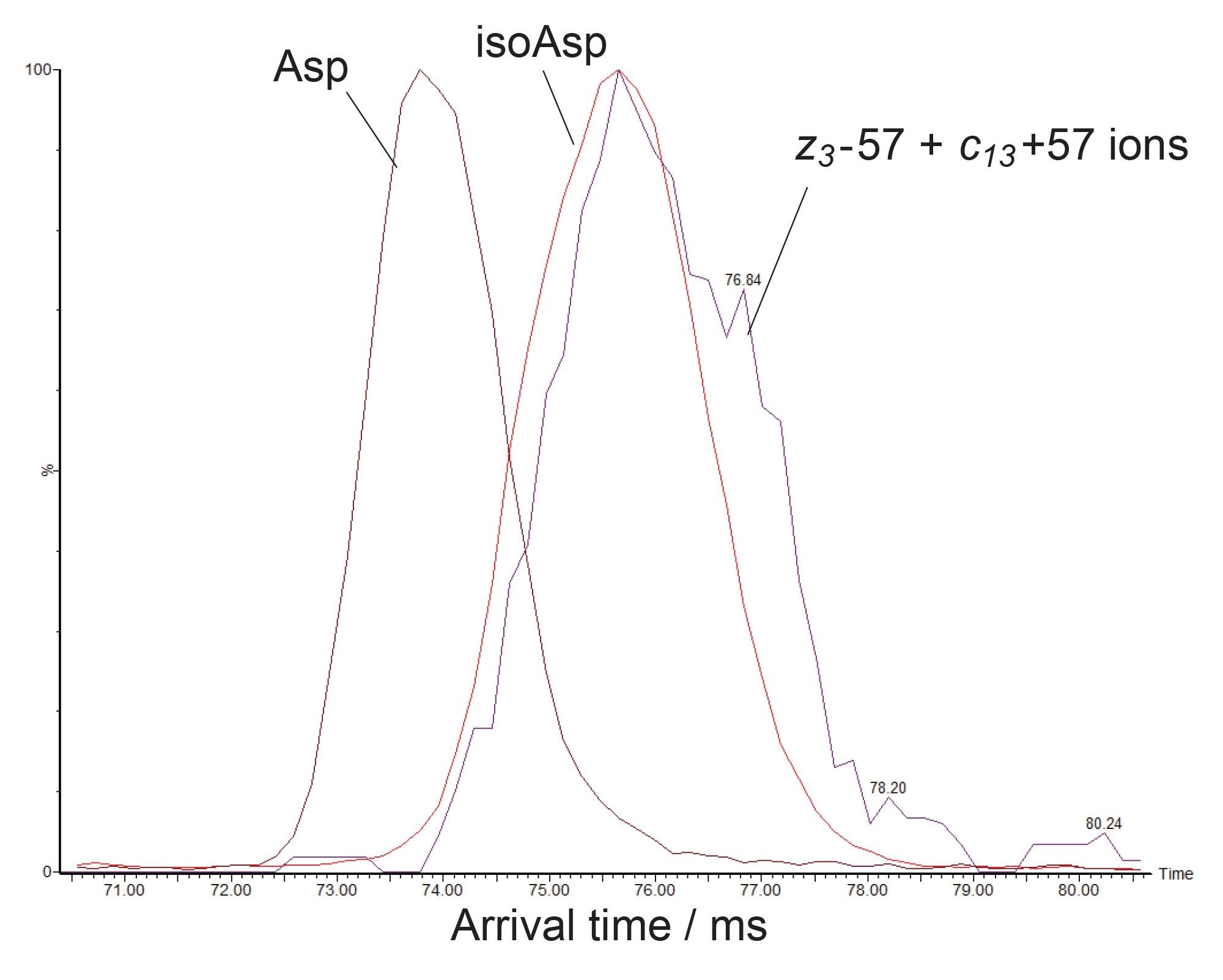
Asp and Isoasp Isomers are Further Distinguished by Multipass Cyclic Ion Mobility
High resolution ion mobility separations, as afforded by the cyclic ion mobility device, offer an additional means to distinguish isomeric amino acids. The Asp and isoAsp forms of the deamidated peptide T26 above have different structures and so have the potential to be separated by ion mobility. Indeed, thanks to the scalable mobility resolving power of the SELECT SERIES Cyclic IMS these peptides could be separated rapidly in conjunction with chromatographic analysis. The arrival time distributions in Figure 5 shows an eight-pass cyclic ion mobility separation where the Asp isomer is the most mobile (73.5 ms) and the isoAsp isomer in the least mobile (75.7 ms). The power of this analysis is further enhanced by the compatibility of the mobility experiment with ECD fragmentation. The arrival time distribution of the combined intensity of the characteristic z3-57 and c13+57 ions overlays with that of the least mobile species (75.7 ms), confirming that this is the isoAsp isomer.
Confirming the Presence of Leucine and Isoleucine
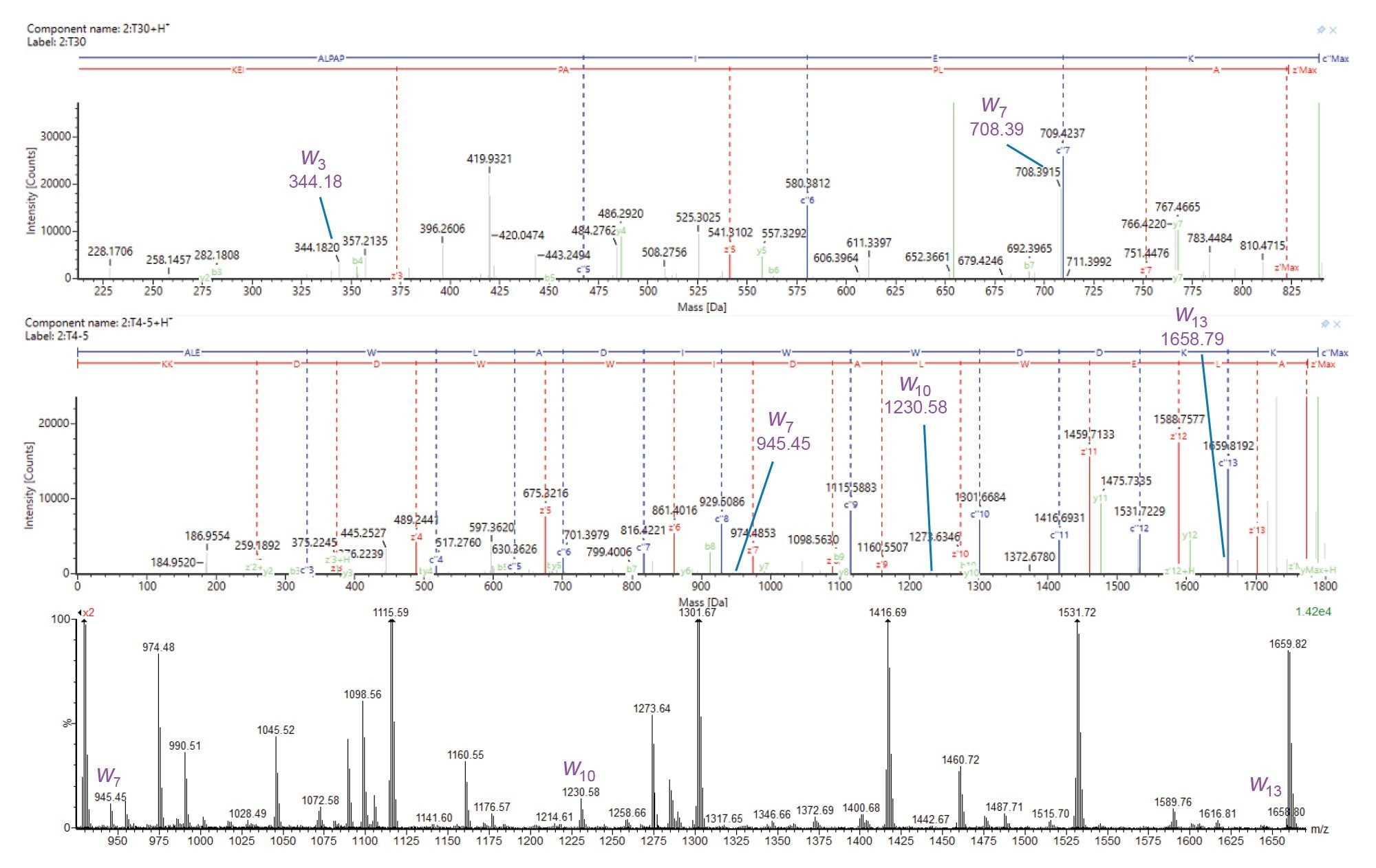
Leucine and isoleucine are natural isomeric amino acids present at significant levels in proteins. Differentiating the two is of potential benefit in clone characterization or in the increasingly popular field of de novo mAb sequencing where the DNA sequence is not determined a priori. Characteristic w ions are formed during ECD of leucine and isoleucine-containing peptides resulting from neutral losses of 43 and 29 Da, respectively (Figure 6).
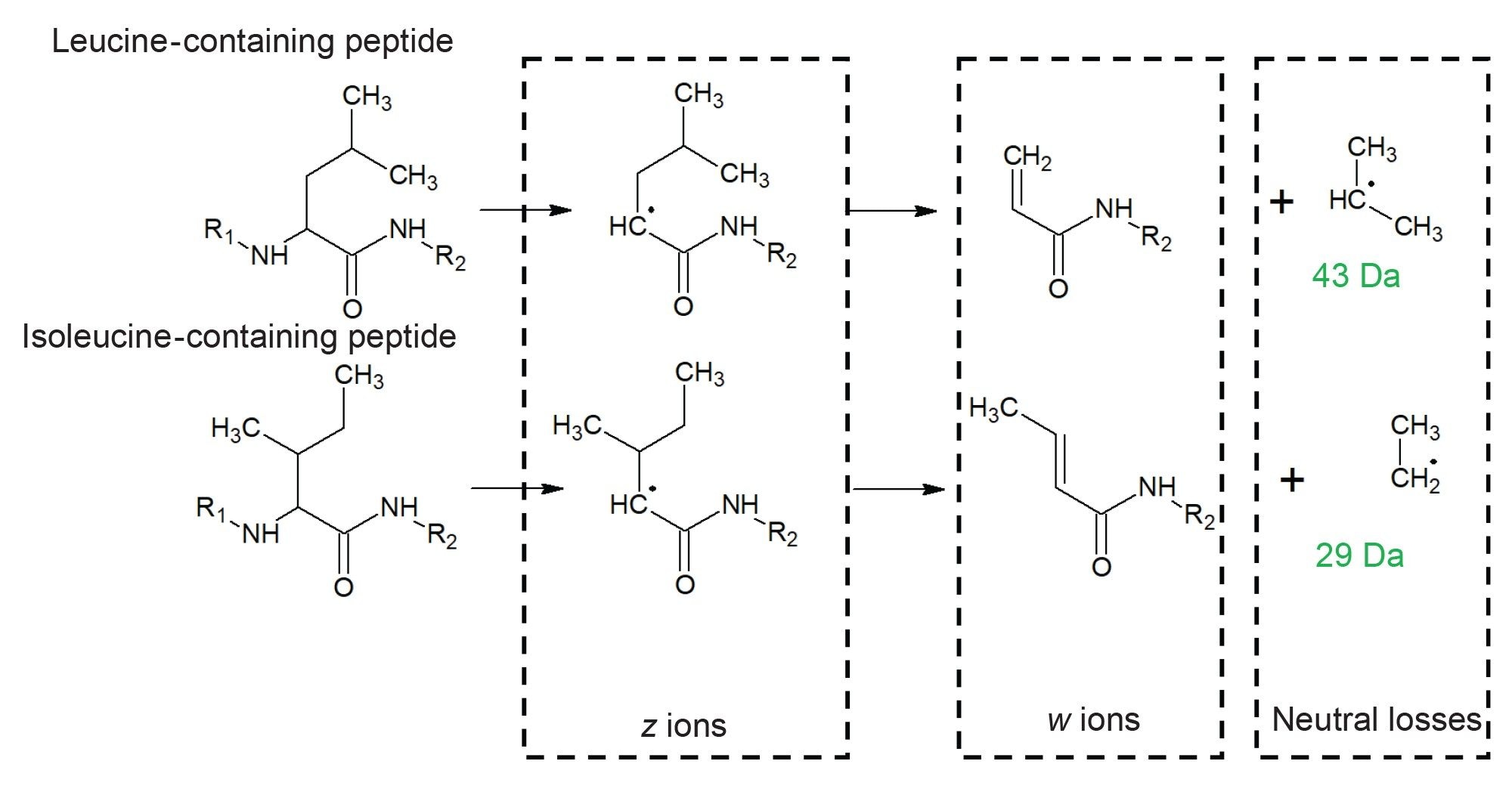
Figure 7 shows ECD product ion spectra of T30 (sequence ALPAPIEK) and T4-5 (sequence ALEWLADIWWDDKK) from the heavy chain of NISTmAb. For T30 we observe ions at 344.18 m/z and 708.39 m/z corresponding to w3 and w7, which confirm isoleucine at position 6 (ALPAPIEK) and leucine at position 2 (ALPAPIEK), respectively. For T4-5, we observe ions at 945.45 m/z (w7), 1230.58 m/z (w10), and 1658.79 m/z (w13), confirming isoleucine at position 8, and leucine at positions 2 and 5 (ALEWLADIWWDDKK).
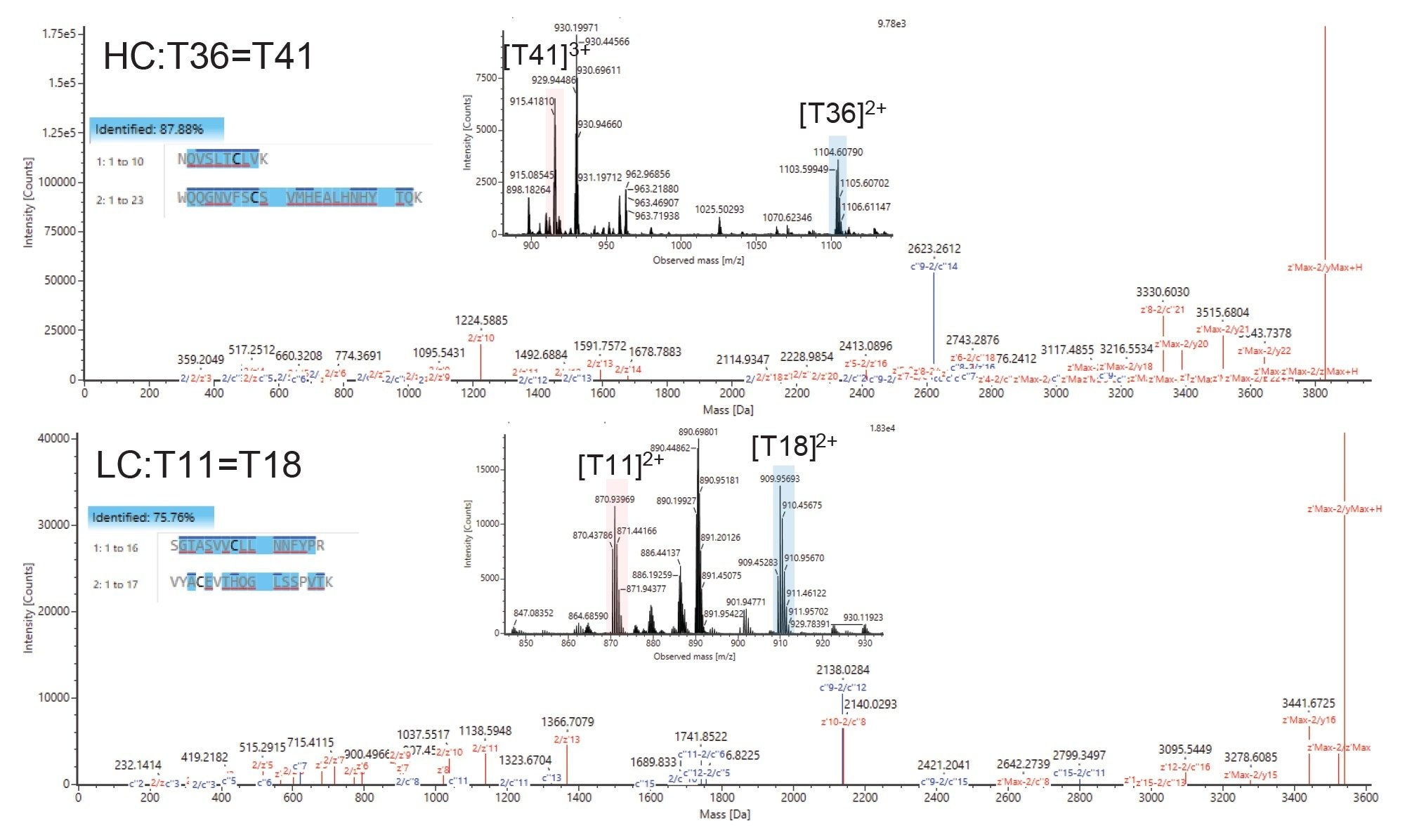
Deeper Characterization of Disulfide-bonded Peptides
Mapping of disulfide bonds within biotherapeutics is also of high importance, as mismatched crosslinks can have a severe effect on higher order structure and stability and, ultimately, drug efficacy and safety. Typically, sequencing of disulfide bonded peptides with CID does not yield fragments close to the site of the cystine crosslink and moreover does not cleave the crosslink itself. Due to their high reduction potential S-S bonds are readily broken after electron capture, meaning the individual peptides are detected in significant abundance as well as their sequence ions (c and z). This enables a deeper level of characterization, yielding crosslinked peptide mass, individual peptide masses, and their sequences, taking it a step further than CID-only workflows.
In Figure 8 we show comprehensive sequencing of the disulfide-containing peptides T36=41 and T11=18 from the heavy and light chains of NISTmAb, respectively. Excellent c and z ion coverage is obtained not just for the crosslinked peptides but for each of the peptides participating in the crosslink, which is not always obtainable by CID methods. The ECD-cleaved intact peptides are also observed allowing unequivocal identification of each of the cross-linked species.
Conclusion
Electron capture dissociation is a powerful fragmentation technique used in the study of proteins which offers exciting potential in the characterization of biotherapeutics. We have shown here an array of examples that demonstrate the utility of ECD in the characterization of several quality attributes that underpin biotherapeutic efficacy, safety, and quality. These include localizing glycosylation sites, confirming leucine and isoleucine residues, distinguishing Asp- and isoAsp-containing isomeric peptides and increasing the confidence in the assignment of disulfide-containing crosslinked peptides. Furthermore, the combination of ECD with high resolution ion mobility separation provides a compelling combination to increase the information obtained from the study of isomeric peptides. Together, these properties of the SELECT SERIES Cyclic IMS instrument reinforce the power of this system for deployment in biopharmaceutical development laboratories.
References
- Zubarev RA, Kelleher NL, McLafferty FW. Electron Capture Dissociation of Multiply Charged Protein Cations. A Nonergodic Process. J Am Chem Soc. 1998 Apr 1;120(13):3265–6.
- Kruger NA, Zubarev RA, Horn DM, McLafferty FW. Electron capture dissociation of multiply charged peptide cations. International Journal of Mass Spectrometry. 1999 Apr 29;185–187:787–93.
- Cournoyer JJ, Pittman JL, Ivleva VB, Fallows E, Waskell L, Costello CE. et al. Deamidation: Differentiation of aspartyl from isoaspartyl products in peptides by electron capture dissociation. Protein Science. 2005;14(2):452–63.
- Kjeldsen F, Haselmann KF, Budnik BA, Jensen F, Zubarev RA. Dissociative capture of hot (3–13 eV) electrons by polypeptide polycations: an efficient process accompanied by secondary fragmentation. Chemical Physics Letters. 2002 Apr 22;356(3):201–6.
- Zubarev RA, Kruger NA, Fridriksson EK, Lewis MA, Horn DM, Carpenter BK. et al. Electron Capture Dissociation of Gaseous Multiply-Charged Proteins Is Favored at Disulfide Bonds and Other Sites of High Hydrogen Atom Affinity. J Am Chem Soc. 1999 Mar 1;121(12):2857–62.
Featured Products
720008287, September 2025