Translating Methods from Helium to Nitrogen Carrier Gas with Atmospheric Pressure Gas Chromatography (APGC™) Source for Multi-Residue Pesticide Analysis
Este é um Resumo de aplicações e, por isso, não inclui uma seção de experimento detalhada.
Abstract
This application brief describes how nitrogen (N2) can be used as an alternative carrier gas to helium (He) in gas chromatography (GC) separations for multi-residue pesticide analysis. The source design of the atmospheric pressure GC (APGC) system allows the use of N2 carrier gas without a loss in performance. To maintain analysis time and chromatographic resolution, a GC column with a smaller inner diameter and thinner film was used. When coupled to a Xevo™ TQ-XS Mass Spectrometer, APGC provides high sensitivity and specificity for the detection of agricultural residues. Here, the analysis of over 200 pesticides was demonstrated with reliable detection for analytes at concentrations as low as 0.5 µg/kg (ppb) in grape matrix with a 1 µL injection volume.
Benefits
- Nitrogen (N2) carrier gas is a readily available, lower cost alternative to helium (He) for GC separations
- Equivalent chromatographic separations and run times with N2 carrier gas can be achieved by scaling the GC column dimensions
- The APGC source can use N2 carrier gas without a loss in performance or instrument uptime
- The Xevo TQ-XS Mass Spectrometer with the APGC source is a robust and sensitive analytical tool for the analysis of pesticide residues
Introduction
Helium is the most commonly used carrier gas for gas chromatography (GC) separations. However, the global supply of He is facing a shortage making it difficult or prohibitively expensive to procure He for chromatography labs. 1 The end of the shortage is difficult to predict, which makes the adoption of an alternative carrier gas appealing to maintain lab productivity. Nitrogen is an inexpensive, safe, and readily available alternative carrier gas for GC. The hesitation for adopting N2 as the carrier gas is that it is traditionally perceived as a “slow” carrier gas and can be challenging to implement for vacuum source GC-MS instruments. The optimum linear velocity for N2 is lower than the optimum linear velocity of He. If N2 is operated under its optimal flow conditions using the same column dimensions as are used with He, the analysis time will be 2–2.5 times longer to achieve the same separation efficiency.2 However, appropriately scaling the column dimensions can allow for N2 to be used as the carrier gas with little or no compromise in analysis time or separation efficiency.3 By replacing a 30 m x 0.25 mm x 0.25 µm GC capillary column for one with 20 m x 0.15 mm x 0.15 µm dimensions and the same phase ratio, N2 can be operated at its optimal flow rate without sacrificing analysis time. The scaled column can then be used with the same oven temperature gradient to achieve equivalent GC separations and run times.
The atmospheric pressure GC source utilizes an ionization technique similar to atmospheric-pressure chemical ionization (APCI).4 Inside the source, N2 is used for the initial formation of charged species for APCI. The introduction of additional N2 from the carrier gas flow has no negative effects on the ionization in the APGC source.5 The softer ionization of APCI also has great benefits for sensitivity and selectivity for GC-MS/MS analysis of pesticides.6–8
Results and Discussion
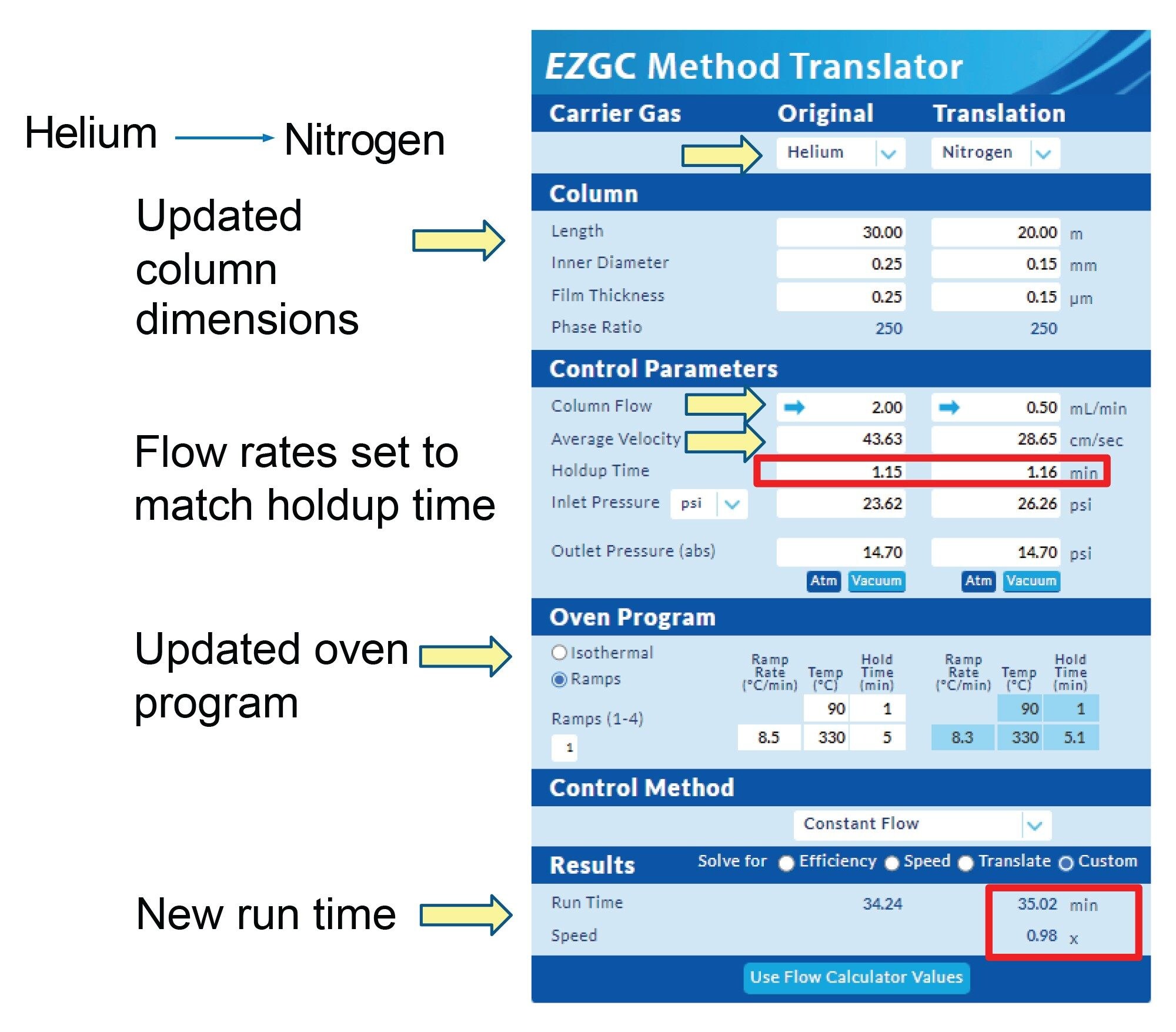
Experiments were performed on a Xevo TQ-XS Mass Spectrometer with an APGC source. The sample chosen for this study was a multi-residue pesticide standard (GC Multiresidue Pesticide Kit from Restek, p/n: 32562) that contains 203 pesticides. The GC method with He carrier gas and MS acquisition method (MRMs) were previously described.8 The Restek EZGC Method Translator tool was utilized for translating the GC method from He to N2 carrier gas with scaled column dimensions (Figure 1). The original method parameters were entered into the translation tool and new column and carrier gas were selected. The column used for the N2 carrier gas method was a Restek Rxi®-5Sil MS 20 m capillary column with 0.15 mm ID and 0.15 µm film thickness. The translation tool gives the option to translate the GC method in terms of efficiency, speed, or a direct translation. To use the same oven program, it is recommended to match the holdup time between methods which required a custom translation. To match the holdup time, the column flow for N2 on the 20 m capillary column was 0.5 mL/min. This flow rate is higher than the theoretical optimum linear velocity for N2, but by using the smaller ID column, the separation efficiency is maintained at the faster flow rate.
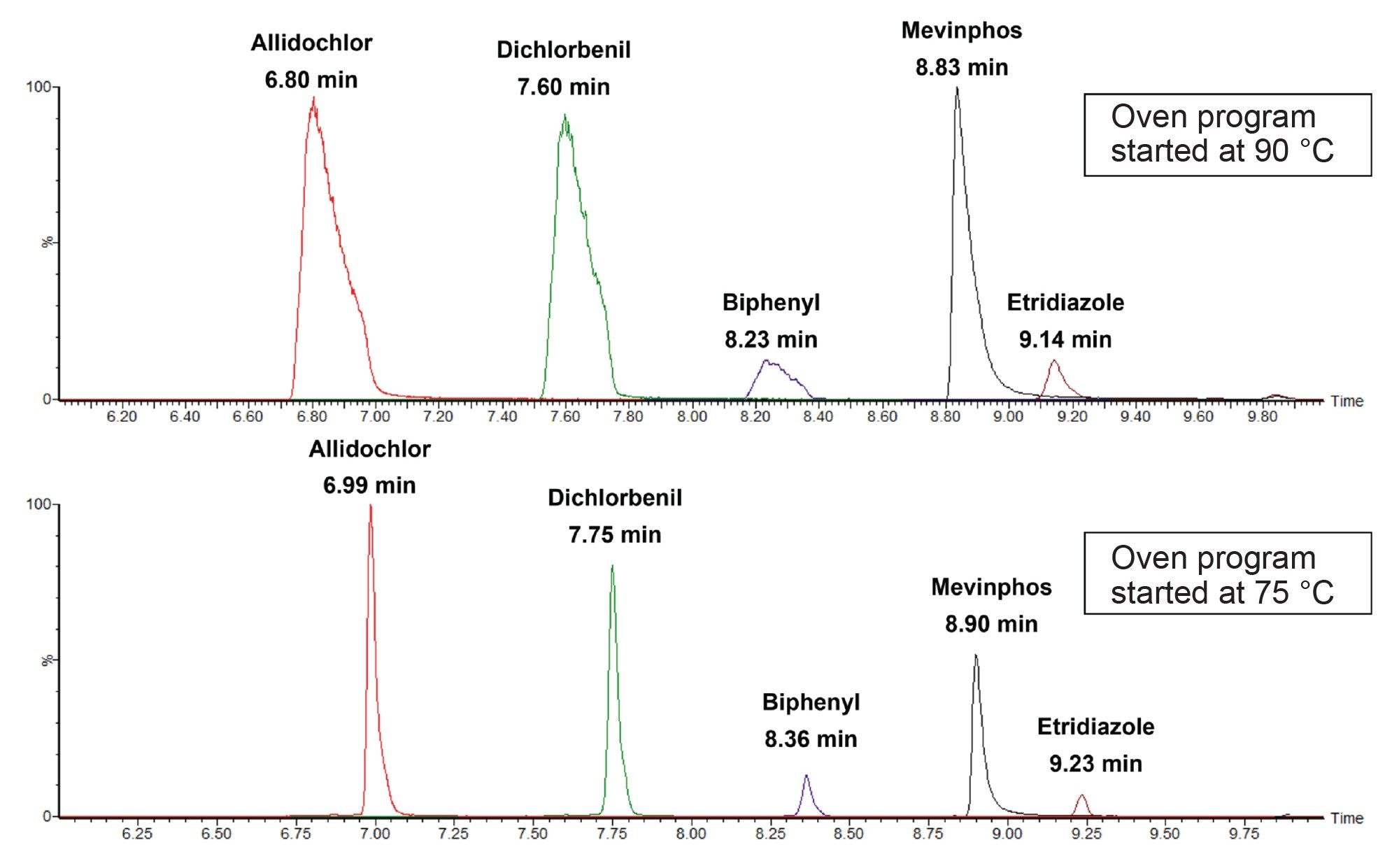
With the translated method, a scaled column and N2 carrier gas, early eluting peaks exhibited poor peak shape when starting the oven program at 90 °C. To address this problem, a solvent focusing step was added to the temperature program. The standard mix was in acetonitrile, which has a boiling point of 82 °C, so the initial temperature of the GC oven was set to 75 °C for 1 minute. The temperature was then ramped from 15.8 °C/min to 106.6 °C to catch up with the original temperature program profile. This initial ramp was during the solvent delay and had no effect on the observed retention times of the pesticides monitored. Figure 2 shows the improved peak shape observed when the solvent focusing step was added.
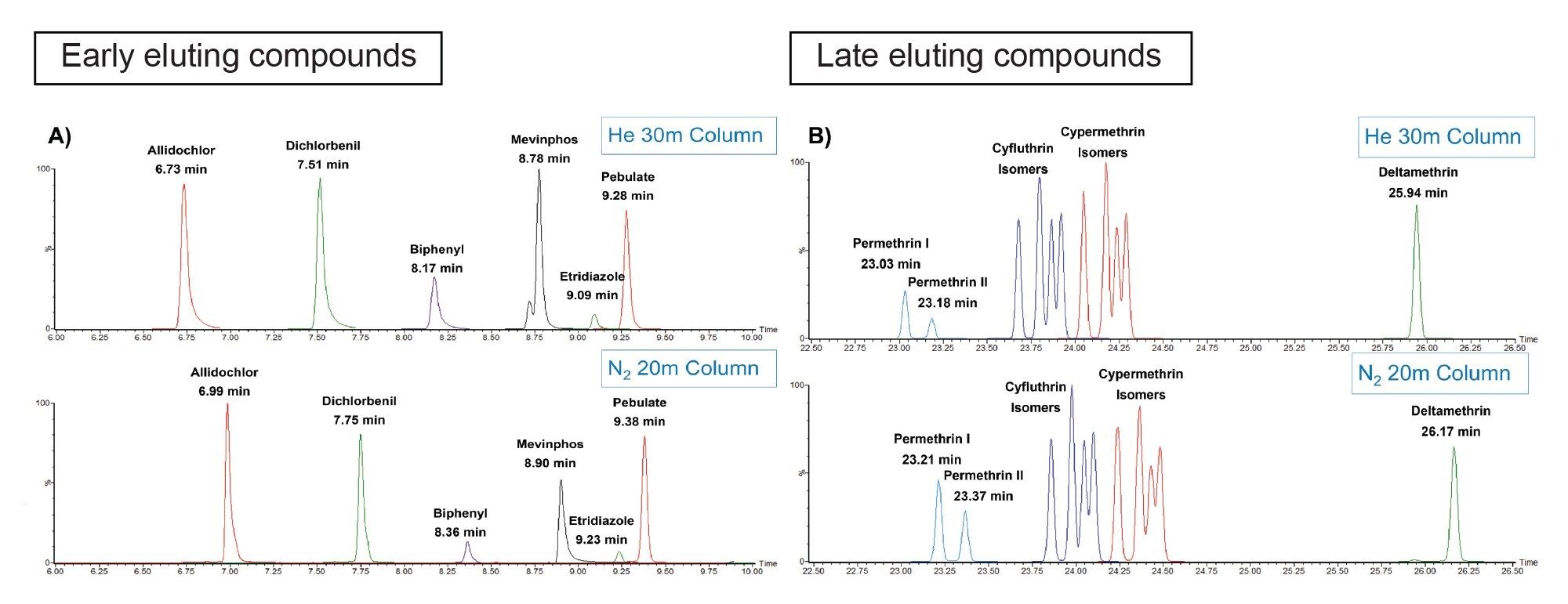
The translated method for N2 carrier gas on the scaled column dimensions of 20 m x 0.15 mm ID as shown in Figure 1 gave a comparable analysis time even with a much lower carrier gas flow rate. By matching the holdup time, the retention times of the compounds from the shorter column were comparable to those observed with He carrier gas on a 30 m x 0.25 mm ID column. Figure 3 shows a comparison of the chromatograms for the early and late eluting compounds from the two different configurations. As the retention times were similar, only minor changes had to be made to the MRM acquisition and processing methods for the 203 pesticides.
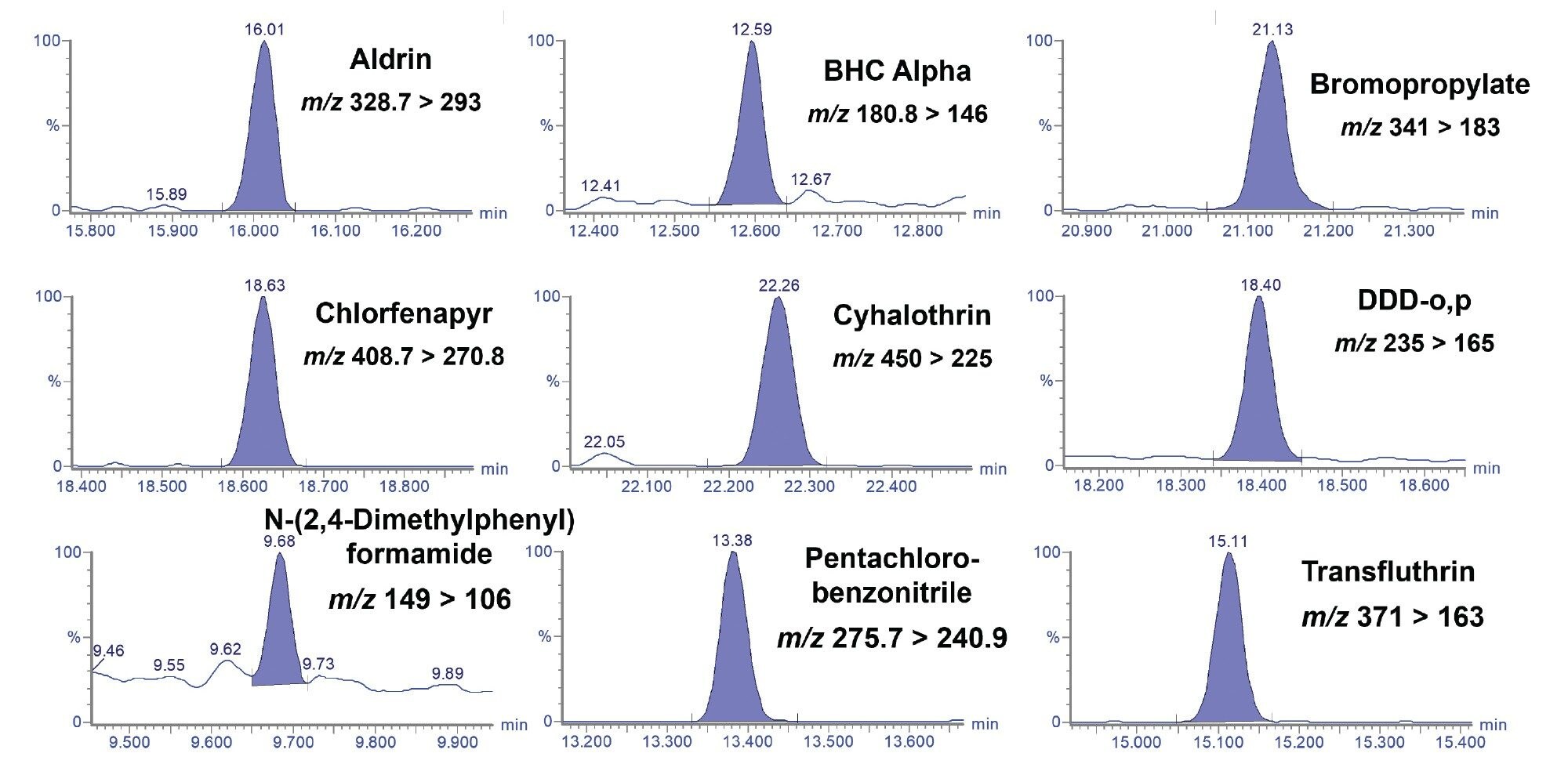
A series of matrix-matched calibration standards were prepared in red grapes that were extracted using the AOAC QuEChERS method.9 The calibration standards were prepared over the range of 0.5–100 µg/kg. The final solvent after QuEChERS extraction was acetonitrile. To improve peak shape, the calibration standards were diluted 1:1 with toluene. A 1 µL splitless injection was used to test the GC-MS/MS method for sensitivity and linearity. Figures 4 shows the chromatograms from a subset of the pesticides analyzed in the grape matrix-matched standard at 1 µg/kg. Bracketed calibration graphs (calibration curves and residuals) from the analysis of a selection of pesticides in grape matrix-matched standards are given in Figure 5.
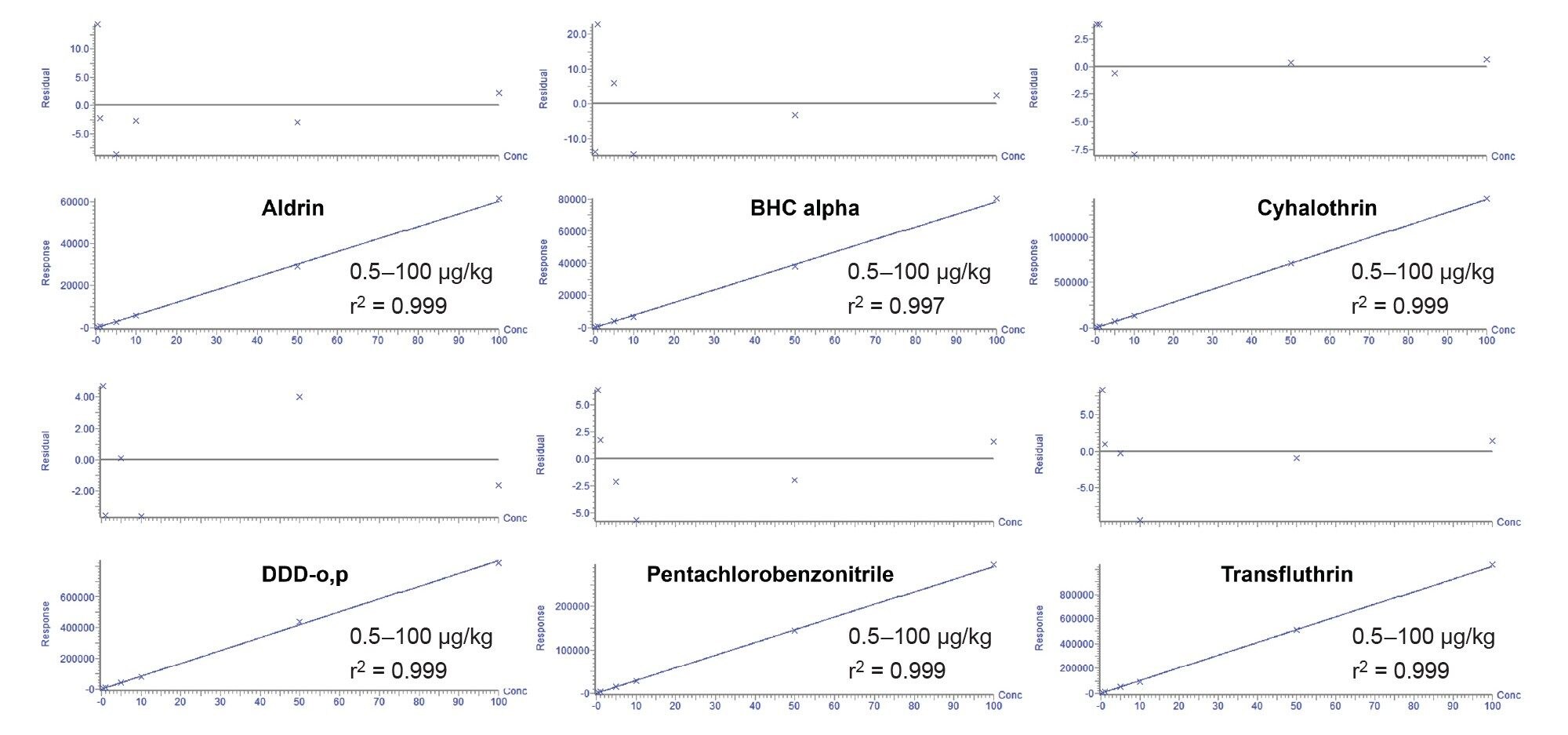
Conclusion
Nitrogen can be used as an alternative GC carrier gas for analysis with the APGC source. By using a column with a smaller inner diameter and thinner film, a comparable temperature program can be used to keep analyses on the same time scale. The translated method with N2 carrier gas on a 20 m GC column was used for the analysis of over 200 pesticides in grapes. The method exhibited very high sensitivity for the analytes measured and the ionization remained robust during the introduction of N2.
References
- Bettenhausen, C. Helium shortages rerun. C&EN. 2022, Volume 100 Issue 6.
- Di Lorenzo, RA, Lobodin, VV, Cochran, J, Kolic, T, Besevic, S, Sled, JG, Reiner, EJ, Jobst, KJ.Fast Gas Chromatography-Atmospheric Pressure (Photo)Ionization Mass Spectrometry of Polybrominated Diphenylether Flame Retardants. Analytica Chimica Acta 2019;1056:70–78.
- Cochran, J, de Zeeuw, J. Changing from Helium and Nitrogen While Maintaining Separation Efficiency and Analysis Time. The Column 2015 Volume 11 Issue 19 https://www.chromatographyonline.com/view/changing-helium-and-nitrogen-while-maintaining-separation-efficiency-and-analysis-time
- Atmospheric Pressure GC (APGC) White Paper 720004771 2013, August.
- Mullin, L, Ladak, A. Harnessing Nitrogen Carrier Gas w/ Atmospheric Pressure Gas Chromatography (APGC) Source. Waters Application Note 720005938 2016, March.
- Portoles, T, Cherta, L, Beltran, J, Gledhill, A, Hernandez, F. Enhancing MRM Experiments in GC/MS/MS Using APGC. Waters Application Note 720004772 2013, August.
- Organtini, K, Cleland, G, McCall, E, Hird, S. UPLC and APGC Multi Residue Pesticide Analysis on a Single Tandem Quadrupole Mass Spectrometer Platform. Waters Application Note 720006013 2017, May.
- De-Alwis JD, Adams S, Hird, S. Determination of Pesticide Residues in Cucumber Using GC-MS/MS with APGC™ After Extraction and Clean-up Using QuEChERS. Waters Application Note 720007654 2022, June.
- QuEChERS Procedure for Multi-Residue Pesticide Analysis White Paper. 720003643 2011, May
720008107, November 2023