
Reaction monitoring is a critical step in the synthesis of new drug candidates. Multiple analyses can be required to assess the progress of a chemical reaction under a variety of different conditions, e.g., different solvents or catalysts. A rapid turnaround in sample analysis for reaction monitoring allows for rapid decision making and increased laboratory efficiency. Within this body of work we have demonstrated that the ACQUITY UPLC H-Class PLUS Binary System coupled to an ACQUITY PDA and ACQUITY QDa Mass Detector is capable of providing separation of atenolol, its intermediate 4-hydroxyphenylacetamide (4-HPA), and the reaction side product 4-hydroxyphenylacetic acid. Achieving these critical separations in a total runtime of 1.2 min while providing information rich mass spectral and UV data allows for easy and confident reaction monitoring, accessible to medicinal chemists with a diverse range of analytical experience.
During SAR (Structure Activity Relationship) studies in the discovery chemistry workflow of pharmaceutical drug design, medicinal chemists optimize the properties of target compounds by introducing different functional groups to known bio-active molecules. The purpose of these experiments is to obtain the most suitable drug candidate with optimal biological activities.
Once a chemical hit is found and verified, optimization of the compound’s desired properties takes place. This step involves an iterative process of synthesis and reactivity measurement of the new compounds to further develop drug candidates into the lead phase.1
Because these reactions may take a long time, chemists need to know as soon as possible if their syntheses are proceeding as desired. This means utilizing measurement capabilities that require minimal sample preparation and provide a fast response given low detection limits. Another advantageous property of the chosen analytical technique might be the ability to measure multiple parameters (e.g., impurity formation and optimum final product yield) simultaneously.2
The purpose of this application note is to demonstrate the utility of the ACQUITY UPLC H-Class PLUS Binary System’s ability to perform short, ballistic gradients for rapid, high throughput chromatographic analysis. Coupled to the orthogonal detection techniques of photodiode array and mass detection, the ACQUITY UPLC H-Class PLUS Binary System provides the synthetic chemist with insight into progress of their reaction, potential side products, and any other issues that might occur in a simple and accessible solution for reaction monitoring workflows.
Chromatographic separation was carried out on the ACQUITY UPLC H-Class PLUS Binary System. Samples were eluted in a 0.7-minute gradient (total runtime 1.2 minutes) with data collected using a PDA Detector and the ACQUITY QDa Mass Detector.
Preparations of atenolol (final product), 4-hydroxyphenylacetamide (4-HPA) (reaction intermediate), and 4-hydroxyphenylacetic acid (4-HPAA) (reaction side product) were prepared to simulate the progress of atenolol synthesis. All standards were sourced from Sigma Aldrich chemicals (Poole, Dorset, UK).
|
LC Conditions |
|
|---|---|
|
LC system: |
ACQUITY UPLC H-Class PLUS Binary System |
|
Detection: |
ACQUITY Photodiode Array (PDA) |
|
Vials: |
Waters Total Recovery Vials |
|
Column(s): |
ACQUITY BEH C18 30 mm x 2.1 mm, 1.7 µm |
|
Column temp.: |
45 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
0.5 µL |
|
Flow rate: |
0.8 mL/min |
|
Mobile phase A: |
0.1% v/v formic acid in water |
|
Mobile phase B: |
0.1% v/v formic acid in acetonitrile |
|
Gradient: |
5 to 95% B/0.7 min |
|
MS system: |
ACQUITY QDa Mass Detector |
|
Ionization mode: |
ESI+ |
|
Acquisition range: |
100–800 Da |
|
Capillary voltage: |
1.5 kV |
|
Collision energy: |
n/a |
|
Cone voltage: |
20 V |
|
MS software: |
MassLynx v4.1 |
During the target compound optimization stage of the discovery workflow, rapid screening is essential for medicinal chemists to accelerate decision making and quickly identify compounds with optimal affinity and selectivity. In all cases chemists need to be able to rapidly assess if the correct product has been synthesized and if a reaction has reached its endpoint in order to quench the reaction for maximum yield.
This stage can be time consuming and labor intensive, therefore it is preferable to have a rapid and reliable means of monitoring experimental progress. In order to demonstrate this workflow, the synthesis of atenolol (Figure 1), was used as a reaction model.
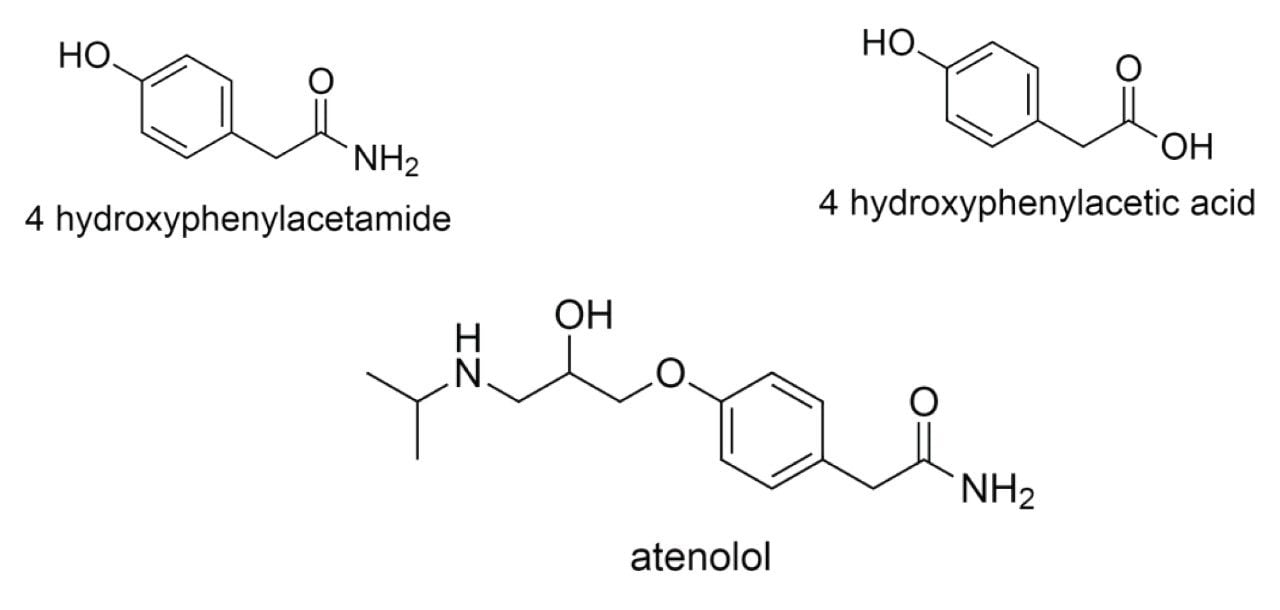
The increase in the formation of atenolol was monitored as well as the decrease in the intermediate 4 -hydroxyphenylacetamide (Figure 2). The reaction by-product 4-hydroxyphenylacetic acid was also observed.
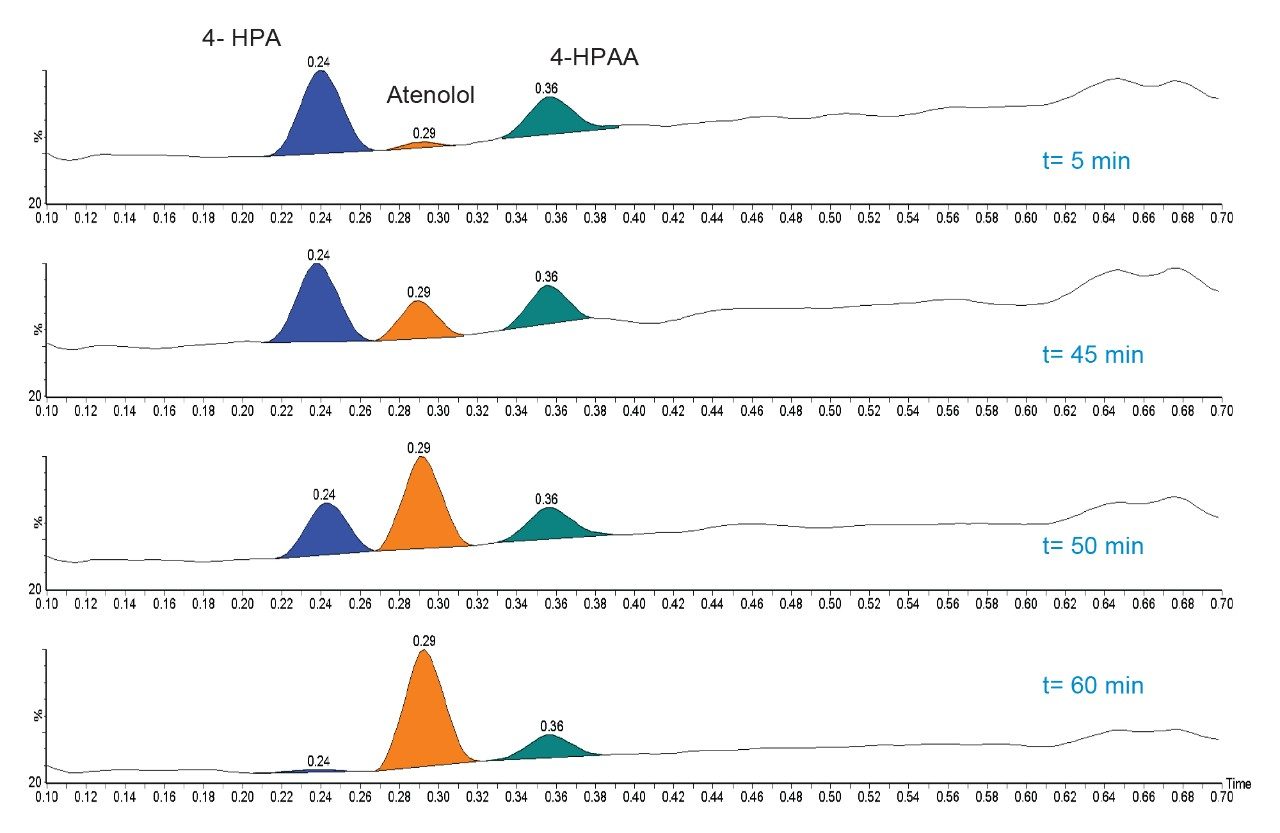
The combination of PDA and ACQUITY QDa detectors provides a simple orthogonal detection system to monitor compounds with different physio-chemical attributes. The 4-HPAA side product gives a strong response in UV with atenolol and 4-HPA providing relatively weak UV responses. With the ACQUITY QDa detection (ESI+ mode) the responses of atenolol/4-HPA are significantly better (Figure 3).
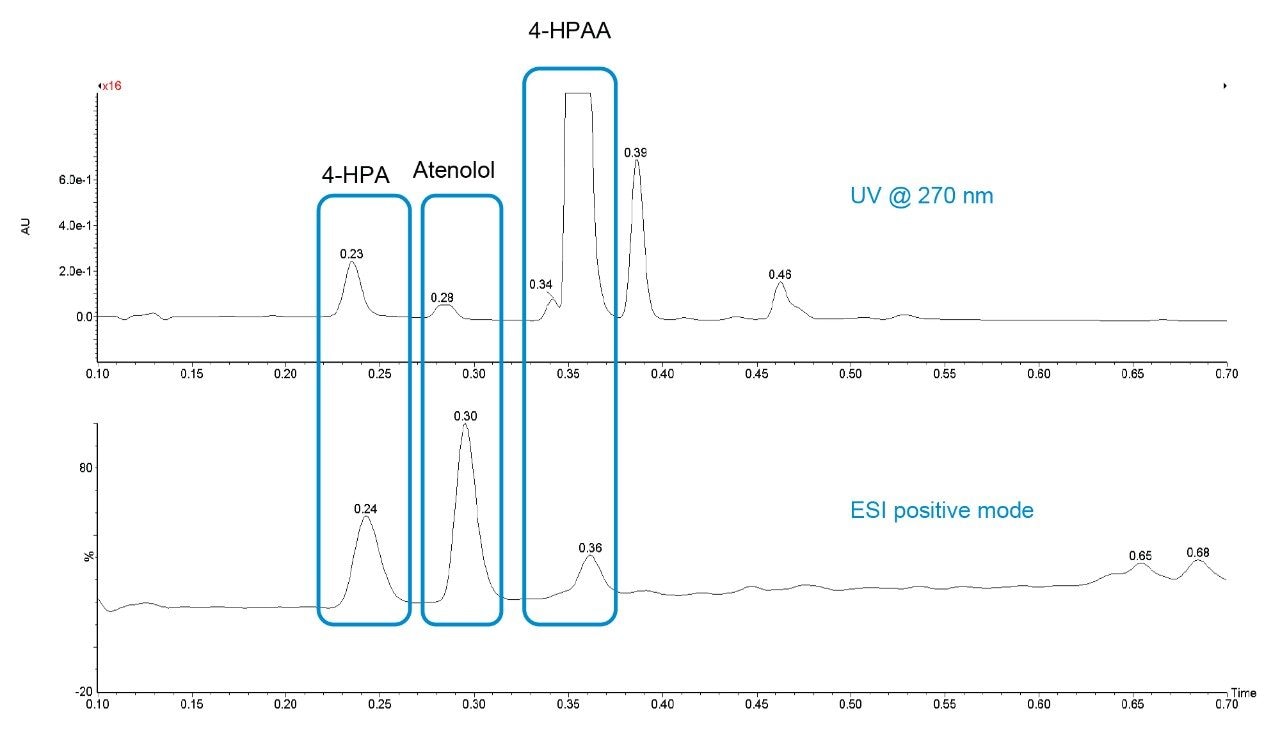
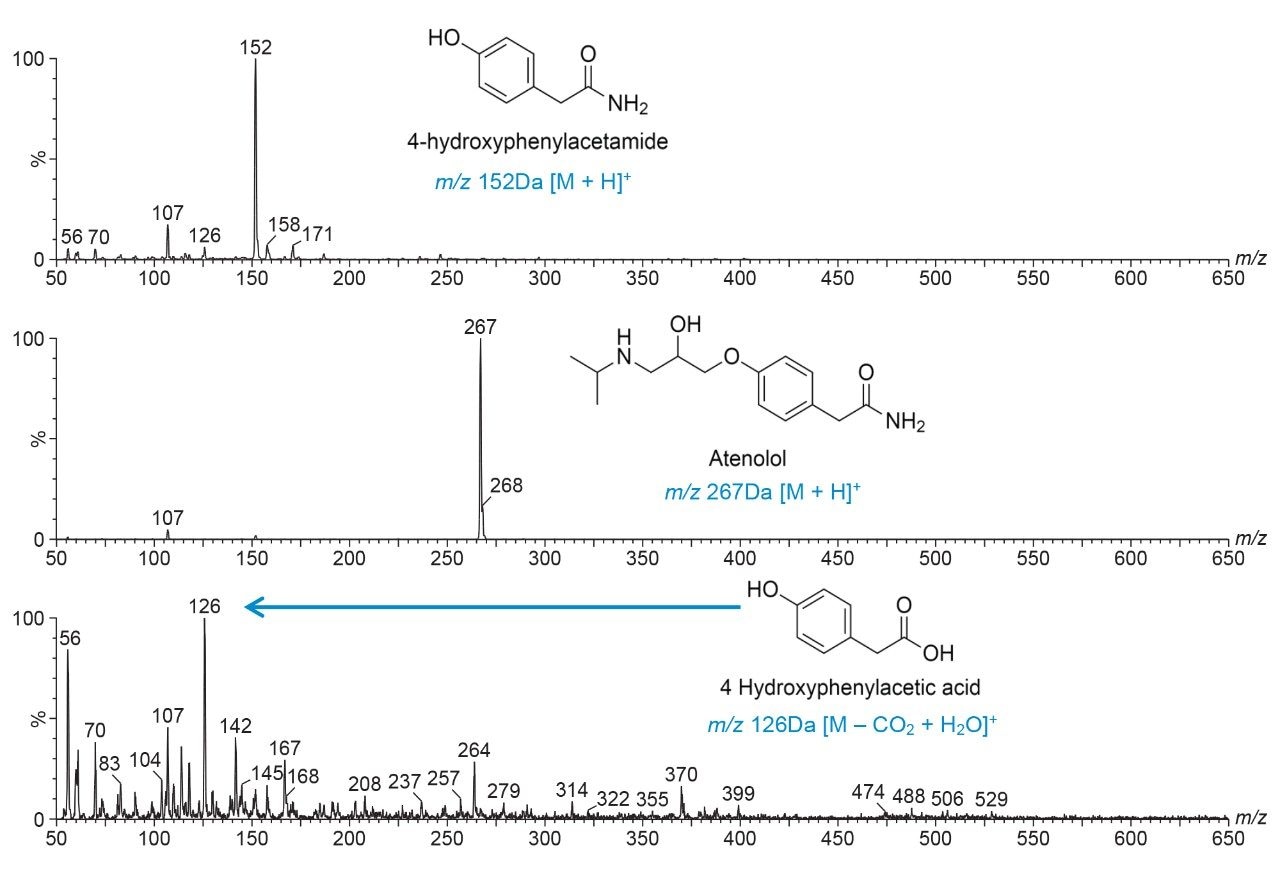
The integrated ACQUITY QDa Mass Detector gives easy access to mass confirmation, which is a significant benefit giving chemists increased confidence of reaction success. Analysis of the mass spectrum of the data generated shows m/z values corresponding to the expected compounds (Figure 4).
By using the ACQUITY UPLC H-Class PLUS Binary System with PDA/ACQUITY QDa detection it was possible for this workflow to achieve UPLC cycle time of 1.2 minutes separating desired reaction product, reaction intermediate and side product compounds.
Mass detection enabled easy mass confirmation of all compounds with the PDA giving additional UV spectral information within the same injection.
The use of this platform gives non-expert mass spectrometry users access to vital mass spectral information during the reaction monitoring progress, thus accelerating the drug discovery process.
720007074, October 2020