Scaling and Migration of a Hydrophilic Interaction Liquid Chromatography (HILIC) Method for Related Compounds of Ribavirin to Modern HPLC Systems
Abstract
Achieving identical chromatographic separation and equivalent results are critical for a successful method scaling across different particle size columns and migration between LC systems. In this work, a HILIC method for the analysis of active pharmaceutical ingredient (API), ribavirin, and related compounds was scaled from a 1.7 µm (2.1 x 50 mm) column to 2.5 µm (3.0 x 75 mm), and 3.5 µm (4.6 x 100 mm) columns with equivalent chemistry. The method was then migrated from an ACQUITY™ UPLC™ H-Class PLUS System to ACQUITY Arc™, Arc HPLC, and Alliance™ iS HPLC systems. Equivalent performance was demonstrated by assessing chromatographic separation, system suitability, and assay results generated by the method on the different systems.
Benefits
- Methods can be successfully migrated between Waters™ UHPLC and HPLC systems
- The Waters Columns Calculator assists in method scaling by calculating operating conditions to generate identical separation and performance
- The Gradient SmartStart feature in Empower compensates for the differences in system dwell volume, eliminating the need for manual adjustments to the gradient table when migrating methods
Introduction
Analytical methods are often developed on sub-2 µm particle size columns to increase the speed of the method development process and reduce analytical run time. These methods generally require a low-dispersion liquid chromatography (LC) system to achieve optimum chromatographic separation. Often methods are transferred to quality control (QC) laboratories that are not equipped with low dispersion systems. In this case, there is a need to scale methods to larger particles designed for use with higher-dispersion HPLCs.1 The receiving laboratory needs to demonstrate equivalent results for the same analysis to ensure product consistency and compliance with the regulatory guidelines.2
Scaling and migration of chromatographic methods between different LC systems can be a challenging task. Often, systems are characterized by different dwell and extra-column volumes, which may result in poor chromatographic separation and peak distortion in gradient methods, subsequently producing inconsistent results for the scaled/migrated assay. The differences in system characteristics must be considered when scaling and migrating methods.3
This work demonstrates the scaling and migration, to modern HPLC systems, of a HILIC method for the analysis of ribavirin and related compounds.4 Ribavirin is an antiviral drug used to treat chronic hepatitis C virus (HCV) infection.5 The method was previously developed on a sub-2 µm particle sized column. In this work, the method is scaled from 1.7 to 2.5 and 3.5 µm particle columns. The method is then migrated from an ACQUITY UPLC H-Class PLUS System (1.7 µm column) to ACQUITY Arc (2.5 µm), Arc HPLC and Alliance iS HPLC systems (3.5 µm). Performance characteristics including chromatographic separation, system suitability, and assay results are examined on each system to measure the success of method scaling and migration.
Experimental
Sample Description
Standard Solutions
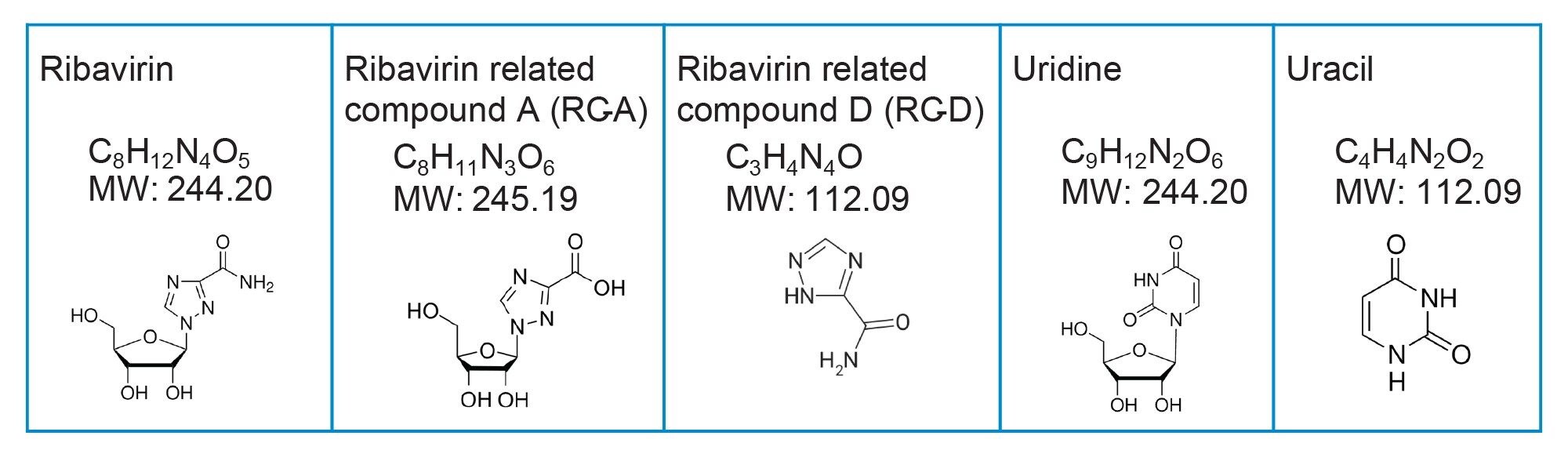
Neat standards of ribavirin and related compounds (Figure 1.) were purchased from Sigma-Aldrich. Individual stock solutions were prepared in 50:50 acetonitrile/water at 1.0 mg/mL. Stock solutions were diluted with 90:10 acetonitrile/water diluent to make a mixture standard solution with ribavirin API at 0.1 mg/mL and related compounds at 10 µg/mL.
Method Conditions
|
Mobile phase A: |
Acetonitrile |
|
Mobile phase B: |
Water |
|
Mobile phase C: |
100 mM ammonium bicarbonate with 1% ammonium hydroxide solution (pH 10) |
|
Column temperature: |
30 °C |
|
Detection: |
UV at 220 nm |
|
Vials: |
LCMS Maximum Recovery 2 mL volume, p/n: 600000670CV |
|
Sample temperature: |
10 °C |
|
Wash solvents: |
Purge/sample/needle: 90:10 acetonitrile/water Seal wash: 10:90 acetonitrile/water |
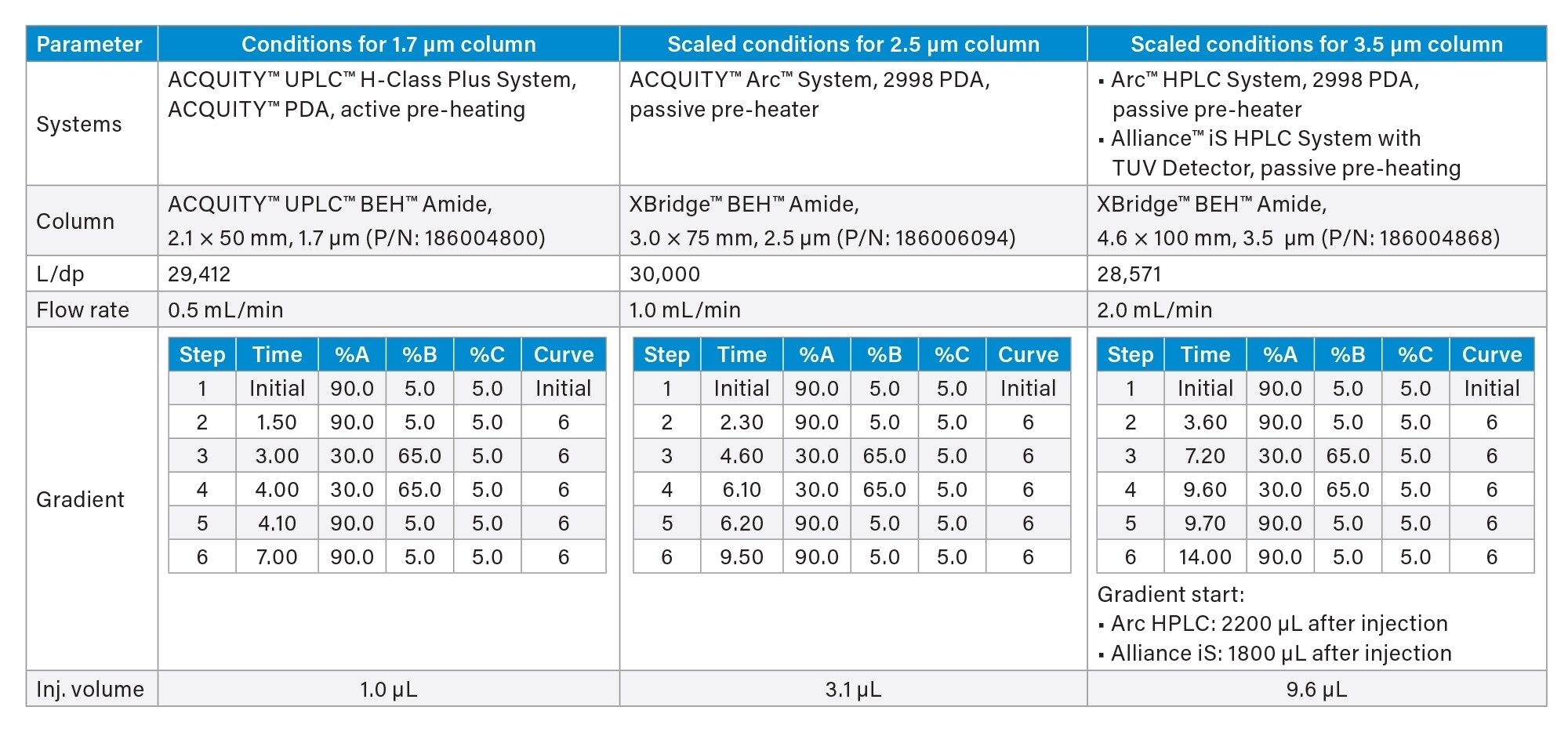
Method conditions used for 1.7 and 2.5 and 3.5 µm particle columns are summarized in Table 1. The flow rate, injection volume, and gradient elution were geometrically scaled using the Waters Columns Calculator.6
Data Management
|
Chromatography software: |
Empower™ 3 Feature Release 5 Service Release 5 (FR5 SR5) Empower 3.8.0 with Alliance iS HPLC System |
Data acquisition and processing performed using Empower Software. Report templates built-in in the Empower project were used to create summary reports of processed data.
Results and Discussion
Considerations: Scaling and Migrating Methods
Scaling and migrating methods between different LC platforms can be impacted by numerous factors. When scaling across different particle sizes, the ratio of column length (L) to particle size (dp) must be the same as the original method to ensure chromatographic separation of the method is preserved. Parameters including flow rate, injection volume, and gradient times are scaled geometrically to generate equivalent chromatographic separation and performance.
Instrument characteristics, including dwell and extra-column volumes, can impact the quality of the chromatographic separation and should be accounted for when migrating methods across different systems.3. Methods operated on sub-2 µm particle columns are generally run on systems with small dwell volumes and low dispersion compared to method that utilize 2.5 to 5 µm particle columns. Operating sub-2 µm particle columns on a large dwell volume and high dispersion system may produce poor chromatographic separation and peak distortion in gradient methods. This may generate different results for the same assay. The procedure for measuring dwell volume of an LC system is described in a white paper.7
Case Study: Ribavirin and Related Compounds
In this work, a HILIC method for ribavirin and related compounds was scaled from 1.7 to 2.5 and 3.5 µm particle columns using Waters Columns Calculator.6 The original method utilized a 50 mm long, 1.7 µm particle column, resulting in L/dp = 29,412. The method was scaled to 2.5 and 3.5 µm particles 75- and 100-mm length columns, producing L/dp of 30,000, and 28,571 respectively. Operating conditions for methods run using 1.7, 2.5, and 3.5 µm particle columns are listed in Table 1.
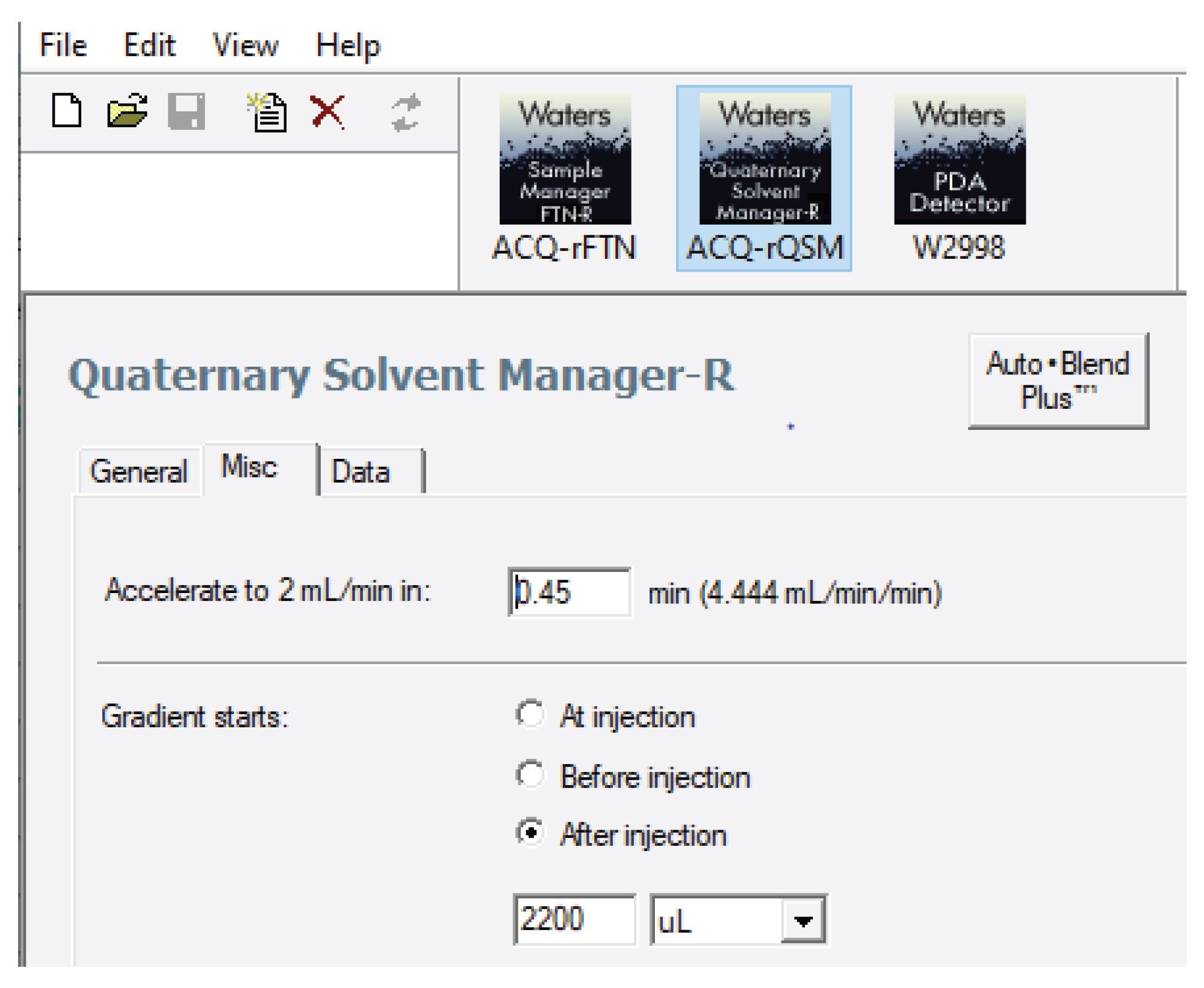
The ACQUITY UPLC H-Class PLUS System has a smaller dwell volume (0.375 mL) compared to the ACQUITY Arc (1.19 mL), Arc HPLC (1.35 mL), and Alliance iS HPLC (1.5 mL) Systems. Therefore, the gradient start for the target methods was adjusted using Gradient StartSmart technology within Empower. An example of gradient start time adjustment for the Arc HPLC System is shown in Figure 2. The gradient was adjusted to start 2200 µL after the injection. The Gradient SmartStart feature compensated for the differences in systems dwell volume, eliminating the need for manual adjustments to the gradient table.
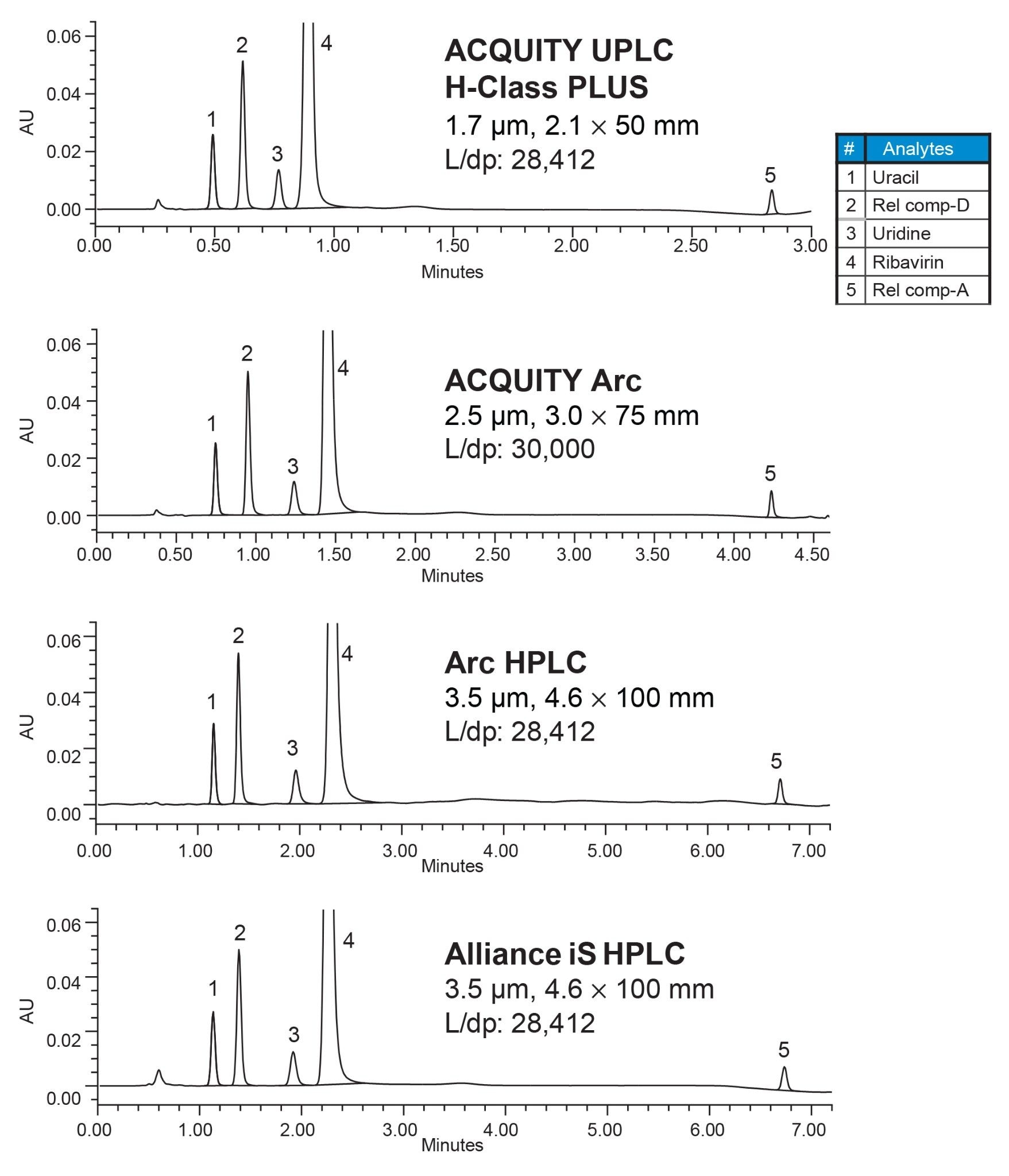
The chromatographic separation produced on the ACQUITY Arc, Arc HPLC, and Alliance iS HPLC systems was comparable to the data on the ACQUITY UPLC H-Class PLUS System (Figure 3). The first four peaks elute under isocratic conditions, while the last peak elutes (related compound A) during the gradient. The separation parameters including the retention times, retentivity factor (k*), and USP Resolution (Rs) between peaks were comparable across all four systems (Table 2).
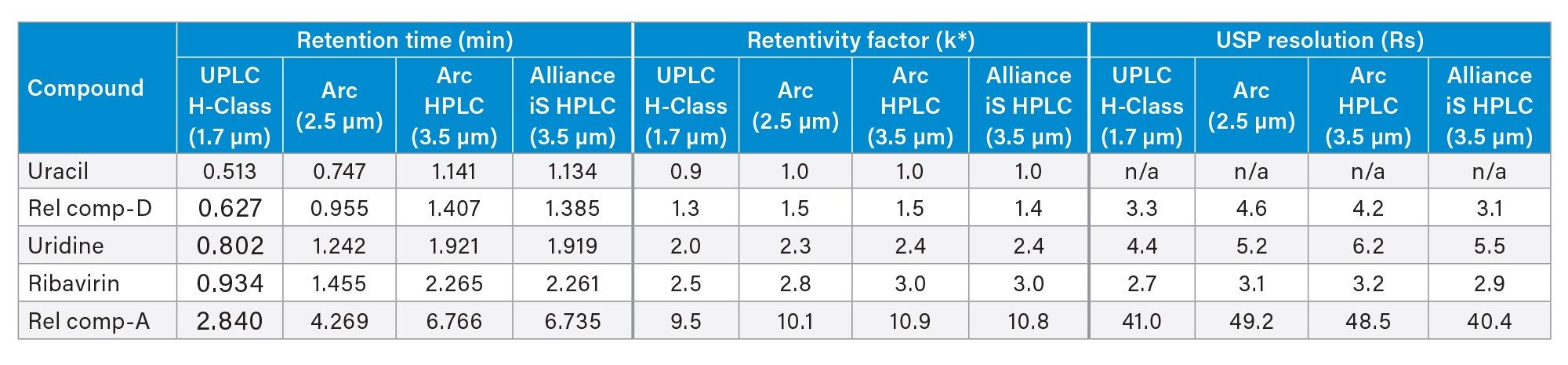
System Suitability
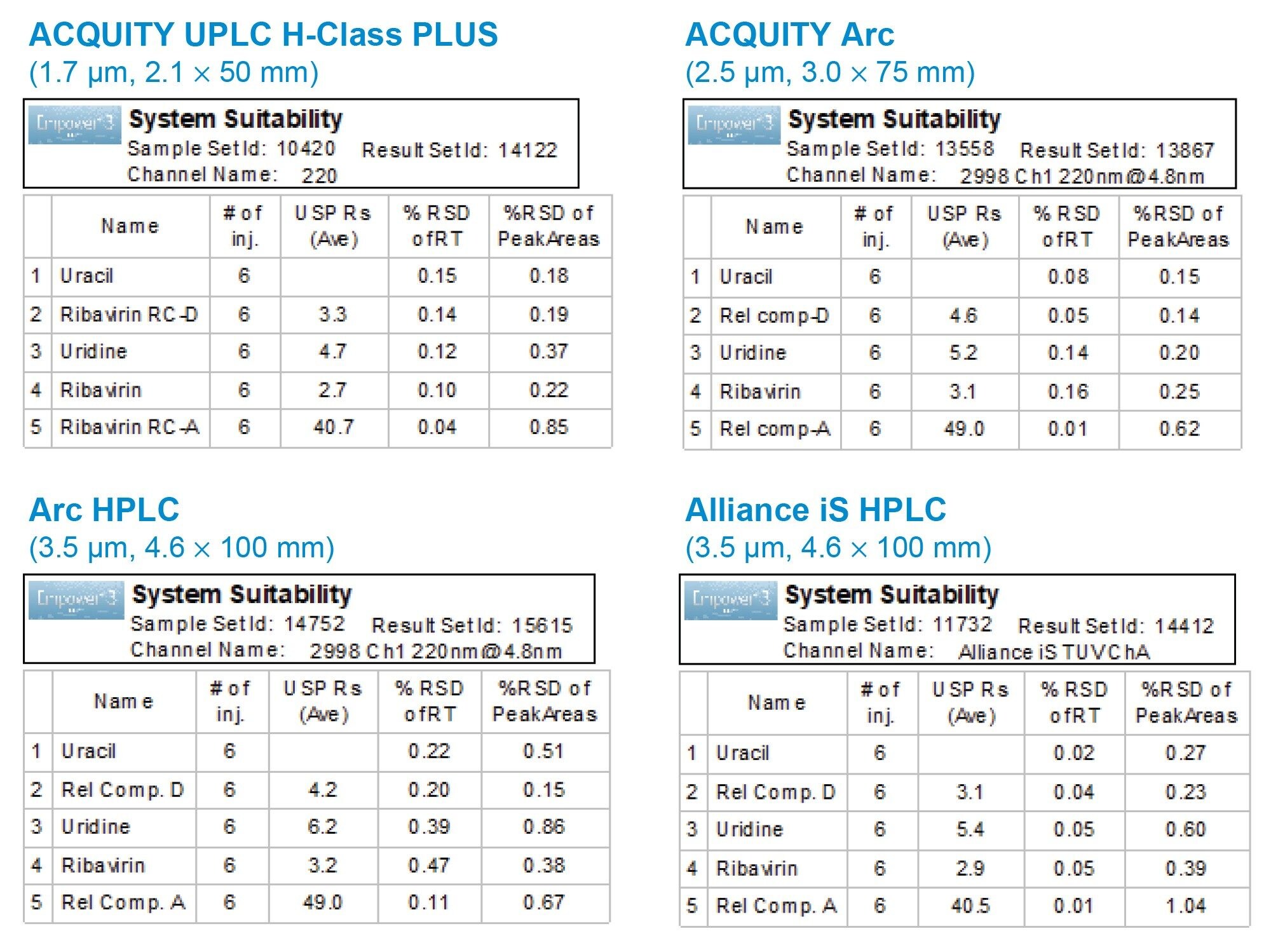
Performance of the method on each system was assessed by measuring repeatability of six replicate injections of a standard solution containing 100 µg/mL of ribavirin API with 10 µg/mL related compounds. The peak areas and retention time repeatability generated by the ACQUITY Arc, Arc HPLC, and Alliance iS HPLC systems were excellent and in agreement with results acquired on the ACQUITY UPLC H-Class PLUS System (Figure 4).
Assay Results for Related Compounds
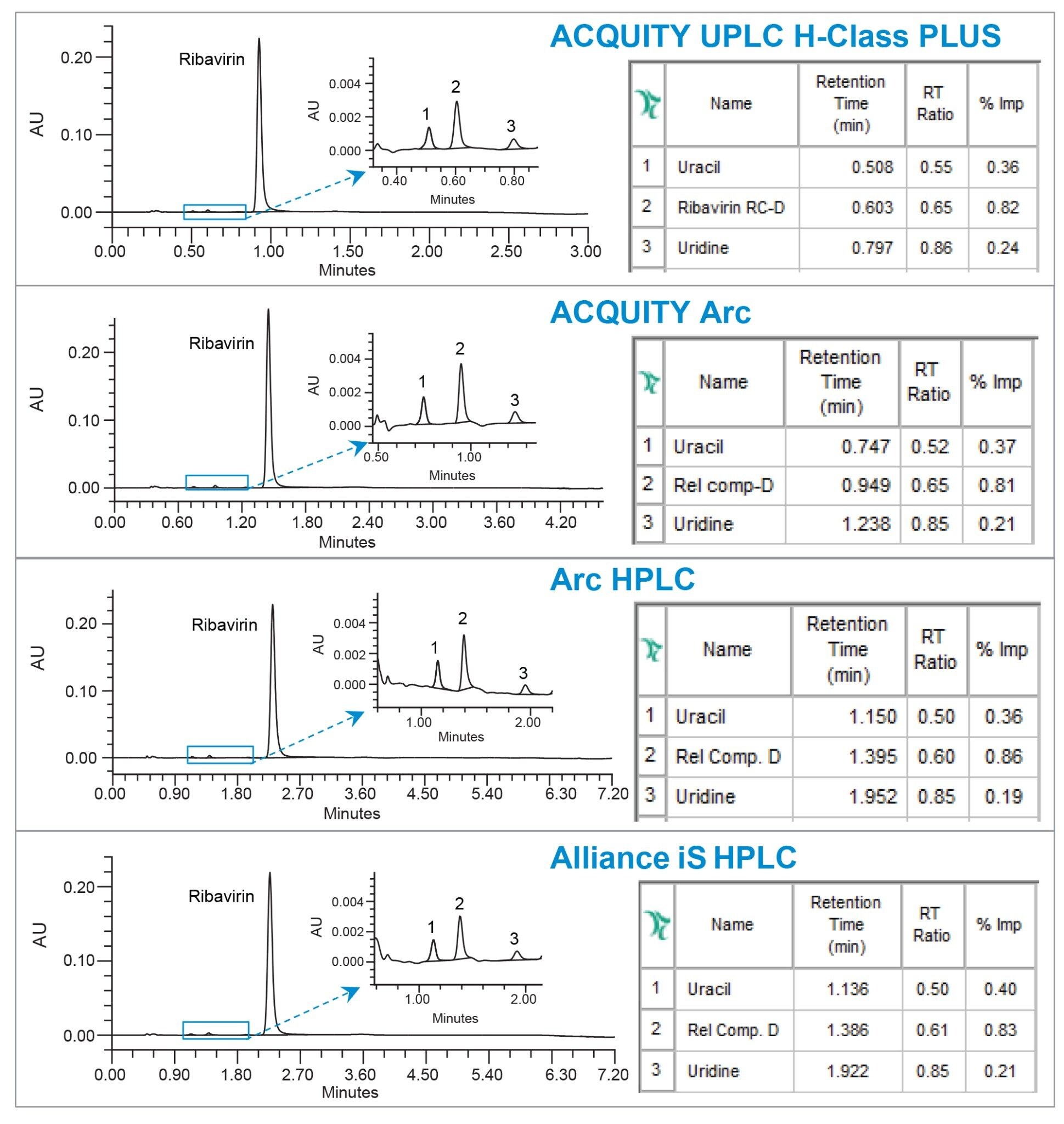
To demonstrate performance of the method for assay analysis of related compounds content (% impurity) an API sample solution was spiked with related compounds and run on different systems with the appropriate column dimensions. The assay results were calculated by comparing the peak area of each related compound to the area of the API, performed using Empower Software. The assay results generated by the ACQUITY Arc, Arc HPLC, and Alliance iS HPLC systems were in agreement with the results produced on the ACQUITY UPLC H-Class PLUS System (Figures 5). The relative retention times (RRT values), calculated using Empower Software by comparing retention of each related compound to the ribavirin API, were comparable across systems.
Conclusion
A HILIC method for the analysis of ribavirin and related compounds was successfully scaled from 1.7 µm to 2.5, and 3.5 µm particle columns and migrated from an ACQUITY UPLC H-Class PLUS System to higher dispersion LC Systems, including ACQUITY Arc, Arc HPLC, and Alliance iS HPLC. The target systems generated equivalent chromatographic separation, system suitability, and assay results compared to the data produced on the ACQUITY UPLC H-Class PLUS System.
System characteristics including dwell and extra-column volumes can impact the quality of the chromatographic performance and must be accounted for to increase the success of method scaling and migration across different LC platforms.
References
- Dong MW. Ultrahigh-Pressure Liquid Chromatography, Part III: Potential Issues: This Installment on Ultrahigh-Pressure Liquid Chromatography (UHPLC) Review the Potential Problems That May Be Encountered Using HPLC Systems and Methods, and Proposes Strategies for Their Migration. LC-GC North America, 35–11, 2017.
- USP General Chapter 〈1224〉, Transfer of Analytical Procedures. United States Pharmacopeia USP 43-NF 38, official prior 2013.
- Hong H, McConville PR. Dwell Volume and Extra-Column Volume: What Are They and How Do They Impact Method Transfer?. Waters White Paper, 720005723EN, 2018.
- Berthelette KD, Walter T, Naughton S, Turner JE, Zampa N. Comprehending COVID-19: Fast Analysis of Ribavirin and Related Compounds Using Hydrophilic Interaction Chromatography. Waters Application Note, 720006957, 2020.
- Ribavirin: https://medlineplus.gov/druginfo/meds/a605018.html
- Waters Columns Calculator. https://www.waters.com/waters/support.htm?lid=134891632&type=DWNL
- Waters Corporation, Protocol for Gradient Delay (Dwell Volume) Measurement. Waters Applications Notebook, 720004313EN, p. 67–69, 2013.
720008255, February 2024