Small molecule anti-viral and anti-inflammatory drugs are currently being repurposed by clinical researchers in the fight against COVID-19 caused by SARS-CoV-2. Dosing studies are typically required during the research phase, with pharmacokinetics (PK) and pharmacodynamics (PD) being key components of this process. A method for exploratory examination of these drug candidates is required to help facilitate this work. LC-MS/MS is a quantitative methodology that meets this need by measuring multiple drugs in a single run and providing quick turnaround times.
A method for the analysis of favipiravir, remdesivir (GS-5734), GS-441524, chloroquine, hydroxychloroquine, desethylchloroquine, lopinavir, ritonavir, and dexamethasone in plasma was developed using 50 µL of sample. The sample was precipitated with an internal standard containing solution, and supernatant was diluted prior to injection on an ACQUITY UPLC I-Class/Xevo TQ-S micro IVD System. Separations were performed with the CORTECS T3, 2.1 mm x 50 mm, 2.7 µm Column using gradient elution and a mobile phase comprised of ammonium formate, formic acid, and methanol.
The method was shown to be analytically sensitive, linear, precise, and accurate, with reproducible extraction efficiency and matrix effects across the associated analyte ranges. Sample stability in plasma was evaluated and it was deduced that exposure to room temperature should be limited to minimize degradation of samples containing remdesivir.
The method represents a useful starting point for an analytical methodology capable of measuring small molecule anti-viral and anti-inflammatory drugs in clinical research.
To enable a rapid response to the COVID-19 pandemic, a wide range of anti-viral and anti-inflammatory drugs are being evaluated in clinical research trials to help reduce the impact of the SARS-CoV-2 virus by impeding viral fusion and replication, as well as subsequent overstimulation of the inflammatory response. Ascertaining the correct dosing regimens of these repurposed drugs can be particularly important with the pharmacokinetics and pharmacodynamics action of some of the newer, more novel anti-virals still not fully understood in this context.
To help support research studies associated with these investigations, suitable analytical methodologies are required to provide precise and accurate data. LC-MS/MS is a quantitative methodology that meets this need by being able to measure multiple drugs in a single run and providing quick turnaround times.
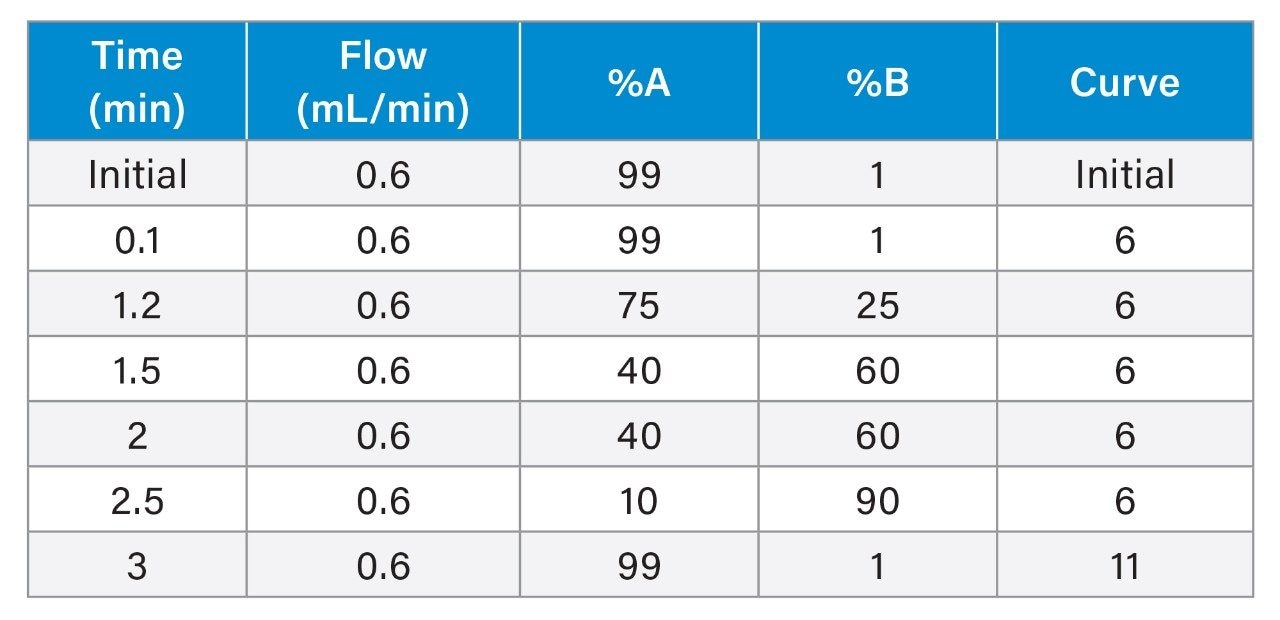
An LC-MS/MS method for the analysis of favipiravir, remdesivir (GS-5734), GS-441524, chloroquine, hydroxychloroquine, desethylchloroquine, lopinavir, ritonavir, and dexamethasone in plasma was developed for clinical research. The method uses a simple protein precipitation followed by injection on the ACQUITY UPLC I-Class PLUS System with detection using the Xevo TQ-S micro Mass Spectrometer. Use of CORTECS 2.7 µm column technology enables rapid separation of the compounds with a run time of four minutes injection-to-injection (Figure 1), while the wide dynamic range of the Xevo TQ-S micro System provides flexibility in the analysis of these compounds during dose escalation studies.
Fifty microliters (50 μL) of plasma was precipitated with 100 µL 30/70 (v/v) methanol/0.1M ZnSO4(aq) containing internal standard. This was thoroughly mixed and centrifuged at 4000 g for five minutes. Fifty microliters (50 µL) of supernatant was diluted with 100 µL mobile phase A prior to injection.
|
LC system: |
ACQUITY UPLC I-Class FL |
|
Column(s): |
CORTECS T3, 2.1 mm x 50 mm, 2.7 µm |
|
Column temp.: |
45 °C |
|
Sample temp.: |
8 °C |
|
Injection volume: |
15 µL |
|
Mobile phase A: |
10 mM Ammonium Formate, 0.2% Formic Acid in Water |
|
Mobile phase B: |
Methanol |
|
Run time: |
3.3 minutes |
|
MS system: |
Xevo TQ-S micro |
|
Ionization mode: |
ESI+ |
|
Acquisition mode: |
MRM (Multiple Reaction Monitoring) See table for details |
|
Capillary voltage: |
0.50 kV |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
500 °C |
|
Cone gas: |
125 L/Hr |
|
Desovlation gas: |
1000 L/Hr |
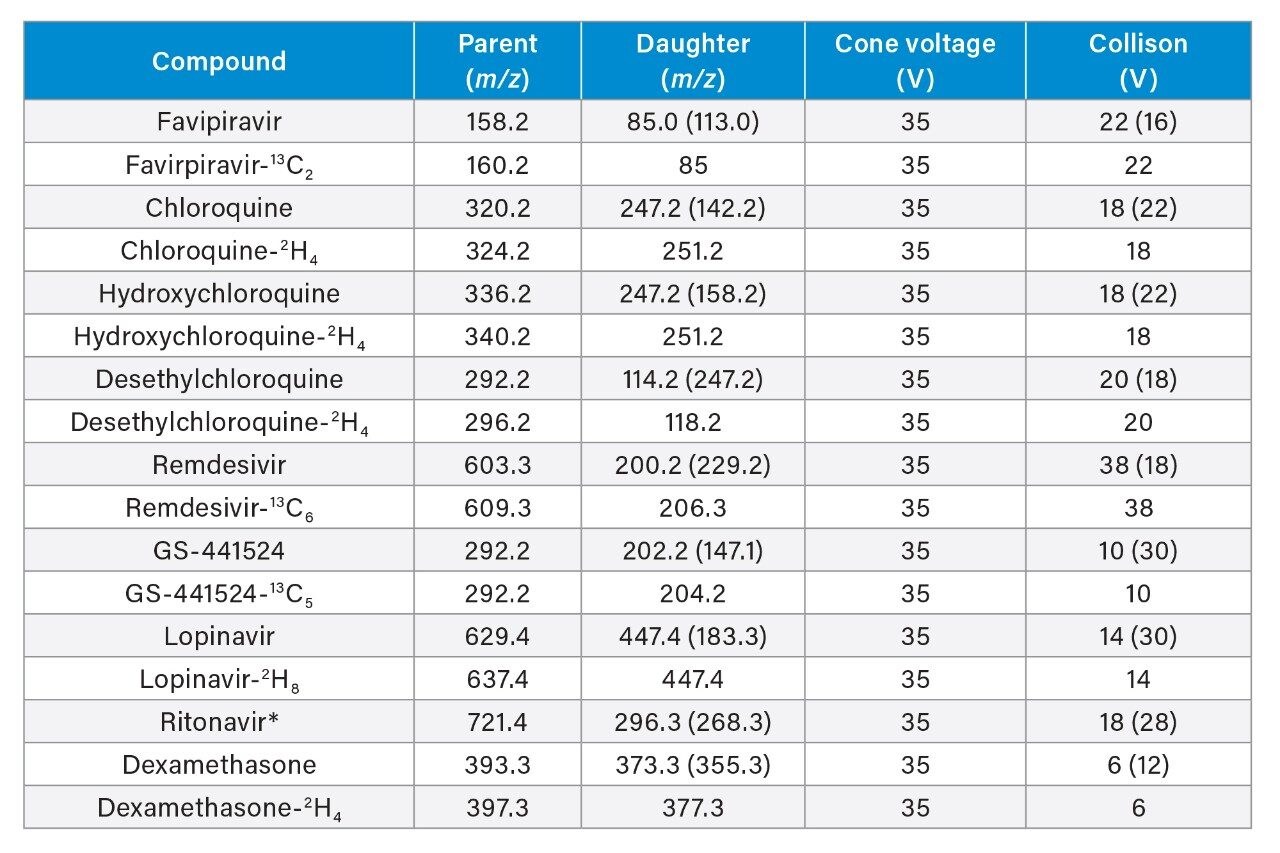
Qualifier parameters are shown in parentheses. (*Ritonavir uses lopinavir-2H8 as its internal standard, due to interference observed during development from high concentrations of ritonavir when using the ritonavir 13C3 label which affected linearity.)
|
MS software: |
MassLynx v4.2 with TargetLynx XS Application Manager |
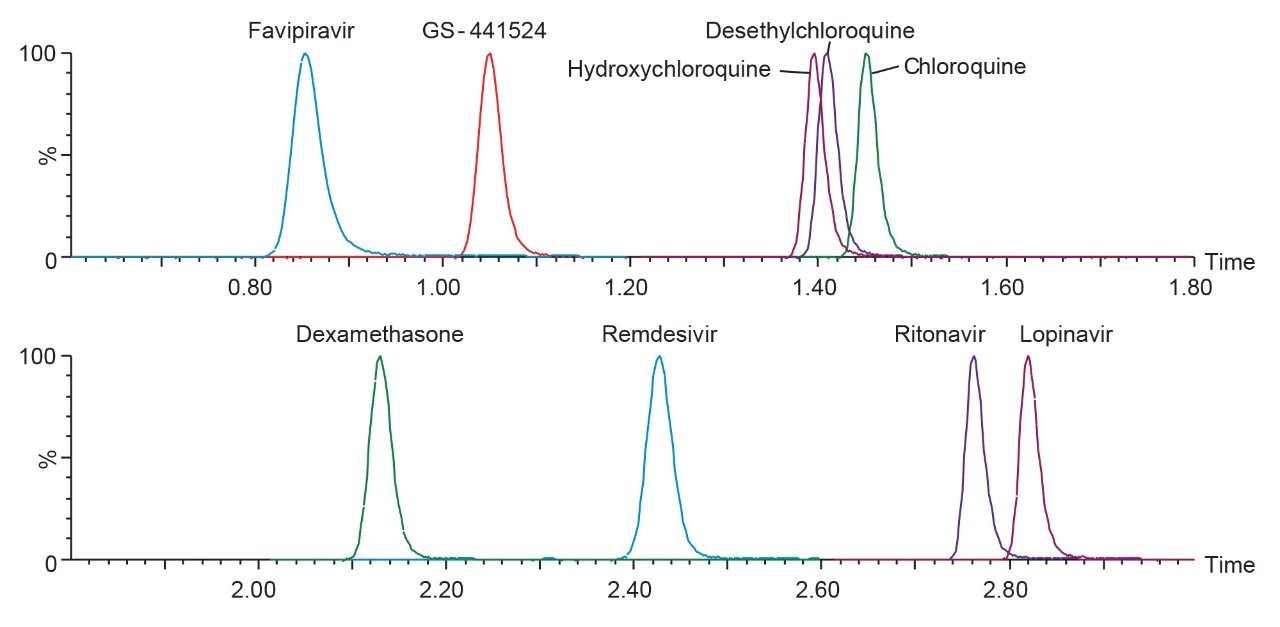
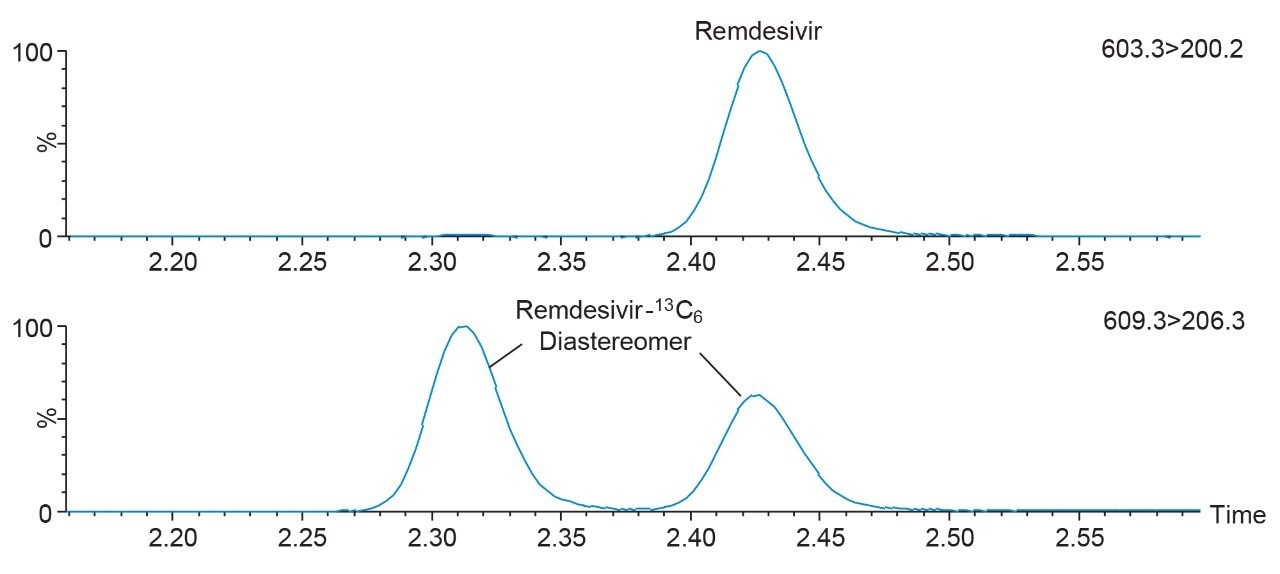
The developed method was evaluated following FDA Bioanalytical Method Validation Guidance for Industry 2018.1 Chromatographic separation was performed using the CORTECS T3, 2.1 mm x 50 mm, 2.7 µm Column. The gradient conditions applied for this method enabled the retention of polar analytes such as favipiravir (0.85 mins) and GS-441524 (1.05 mins), while providing chromatographic selectivity of non-polar compounds such as ritonavir (2.76 mins) and lopinavir (2.82 mins), including separation of the remdesivir-13C6 diastereomer internal standard for accurate quantitation of remdesivir (Figure 2).
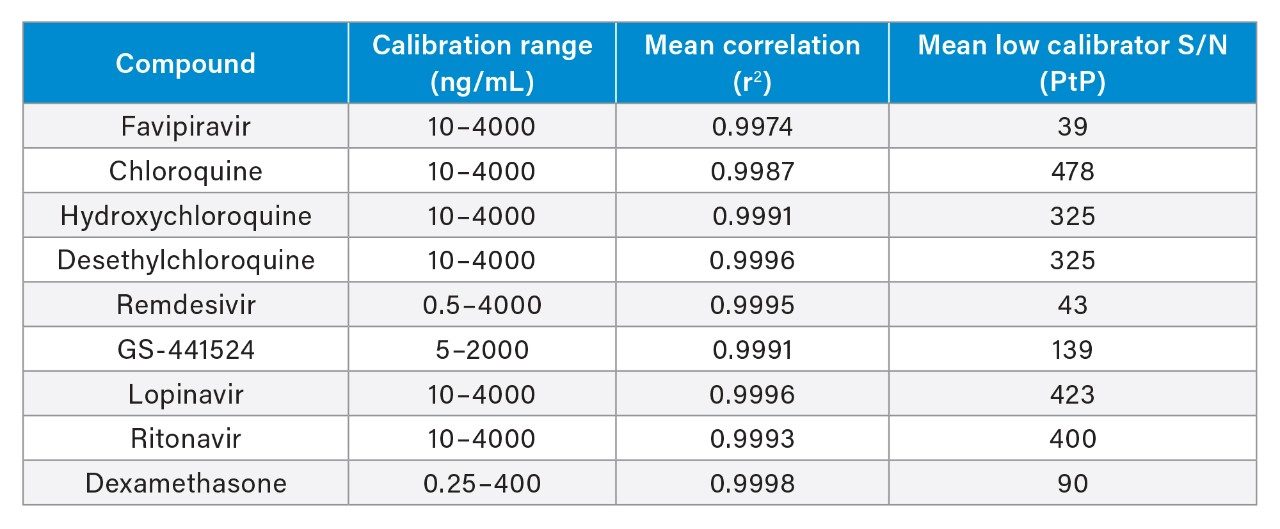
The method was shown to be linear for all analytes, with r2>0.995 for the calibration lines (1/x weighting) across five separate runs, demonstrating the capability of the method to analyze these compounds across a wide dynamic range (Table 1). Analytical sensitivity of the method was demonstrated for these analytes through the assessment of the signal-to-noise ratio (S/N) of the low calibrator samples. S/N was shown to be >25:1 for these low calibrators across five separate runs.
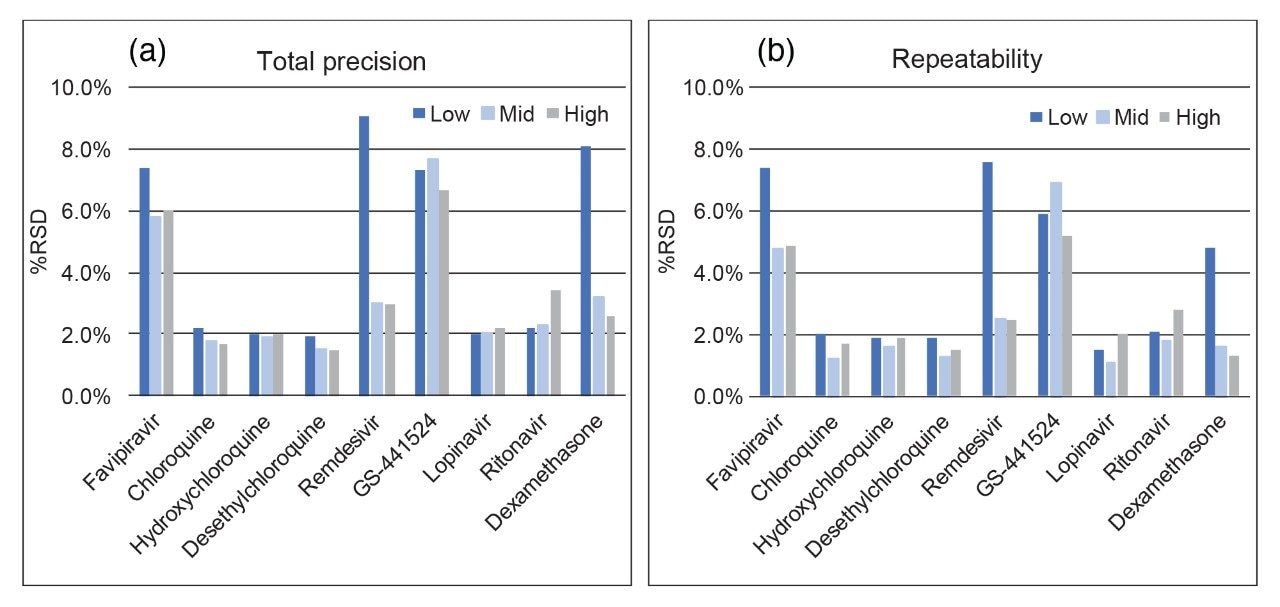
Total precision of the method was determined by extracting and quantifying five QC replicates at three concentrations per day over five separate days (n=30). Repeatability was assessed by analyzing five replicates at each QC level. Low, mid, and high concentrations were 40, 400, and 3000 ng/mL for favipiravir, chloroquine, hydroxychloroquine, desethylchloroquine, lopinavir, and ritonavir; 2, 400, and 3000 ng/mL for remdesivir; 20, 200, and 1500 ng/mL for GS-441524, and 1, 20, and 200 ng/mL for dexamethasone. QC accuracies ranged from 94–108% and total precision and repeatability were ≤9.1% across all analytes and concentrations (Figure 3).
It has previously been established that remdesivir is unstable in plasma at room temperature,2 therefore, room temperature and freeze/thaw stability of the analytes in plasma samples were evaluated to understand the impact of analyte instability during the sample storage and preparation process. The average room temperature stability over two and four hours, and three freeze/thaw cycles at low and high concentrations, are shown in Table 2.
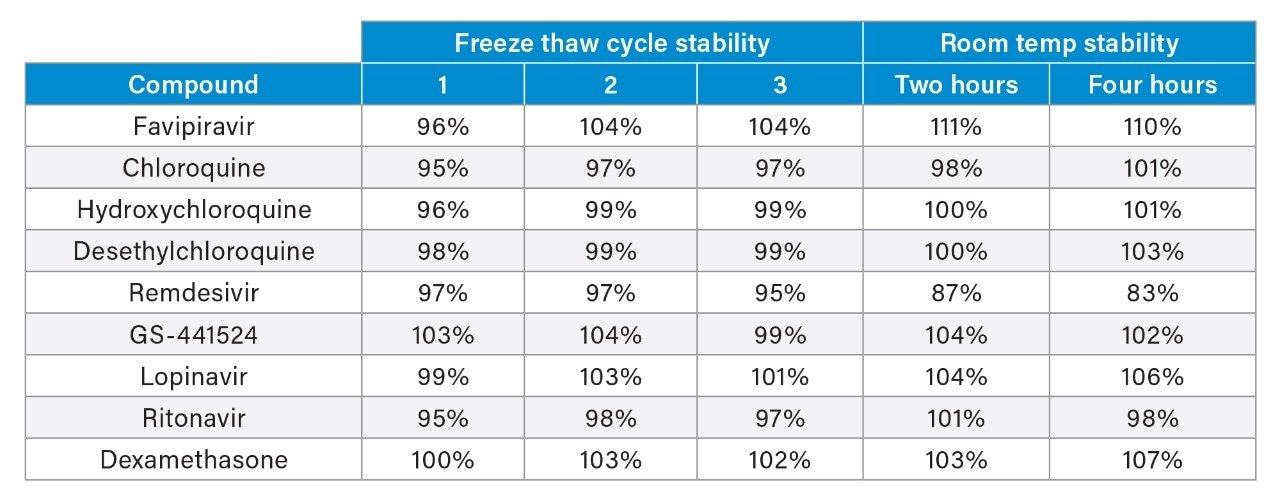
Results indicate exposure to room temperature should be minimized for any samples containing remdesivir. Samples were shown to be stable during three freeze thaw cycle assessments. The stability of extracted samples onboard the autosampler was also investigated and samples were shown to be stable when stored at 8 °C for 124 hours.
Matrix effect investigations for the analytes were performed using individual plasma donor samples (BioIVT, n=6). The matrix factor calculated is shown in Figure 4. Normalized matrix factor calculations based on analyte:internal standard response ratio demonstrated that the internal standards compensated for any of the ion suppression observed and this was reproducible across individual samples.
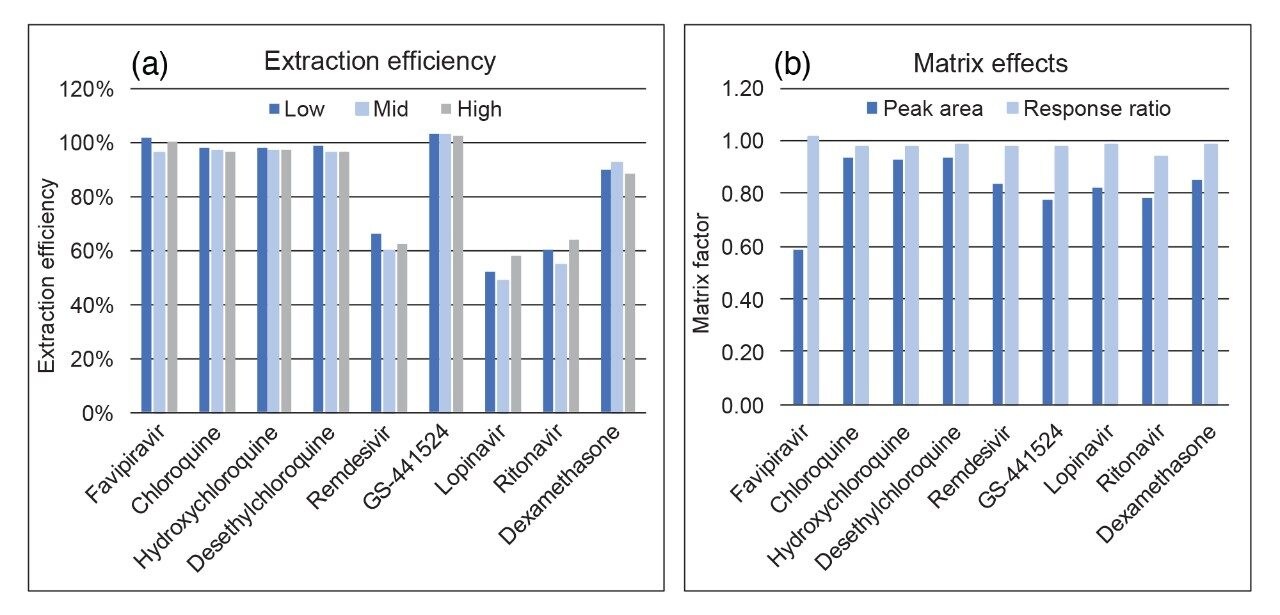
Extraction efficiency was evaluated through the assessment of plasma samples extracted at three concentrations, which were then compared to post-spiked blank plasma samples. Extraction efficiency was shown to be reproducible across the concentrations for each of the analytes with the nonpolar compounds showing lower recoveries (Figure 3b).
A simple and rapid LC-MS/MS method has been developed for the analysis of anti-viral and anti-inflammatory drugs in plasma for clinical research using the ACQUITY UPLC I-Class/Xevo TQ-S micro IVD System.
The method uses a simple protein precipitation to extract the drugs from plasma and chromatography with a CORTECS T3 Column to enable selective and rapid separation of the drugs in the extracted sample. Analytical sensitivity, linearity, precision, and accuracy are excellent with reproducible extraction efficiency and matrix effects.
Clinical research into the impact of these drugs and their metabolites on SARS-CoV-2 is still ongoing. Phosphorylated metabolites of the RNA polymerase inhibitors haven’t been evaluated here and they may be of additional interest, with nucleoside triphosphate of remdesivir representing the predominant metabolite in Peripheral Blood Mononuclear Cells (PBMCs).3 Phosphorylated molecules bind to metals and represent an analytical challenge for robust and reproducible quantification in LC-MS/MS. Chelator additives or the use of high performance surface (HPS) technologies can help bridge the gap in providing a method for robust quantification of phosphorylated molecules in future studies using LC-MS/MS.
720006987, August 2020