
For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
This application brief outlines the quantification capabilities and linear response of the Vion IMS QTof for the analysis of human urine.
The demonstration of Vion IMS QTof linearity of response for metabolic profiling in biomedical research.
Metabolic profiling is a fast-emerging science which provides insight into changes in biochemical pathways in response to disease state or a toxicological insult. It provides insight not only into which metabolites have been up or down regulated, but the rate of these changes. Successful metabolomics methodologies involve the detection and quantification of hundreds or thousand of metabolites in biochemical fluids such as plasma and urine.
Accurate mass LC-MS has become a mainstay of metabolomic research due to its sensitivity, specificity, speed of analysis, and structural identification abilities. In order to provide reliable quantitative data, the mass spectrometry system must provide a linear response over the systemic concentration range of the metabolites.
The Vion IMS QTof is equipped with a novel two-stage Tof detector, QuanTof 2 Technology, allowing the detection of metabolites over a wide dynamic range. Here we demonstrate the linear dynamic range of the Vion IMS QTof Mass Spectrometer for metabolomics analysis.
The linear dynamic range of the Vion IMS QTof was evaluated in urine spiked with the Waters LC-MS test mix, containing seven non-endogenous compounds diluted to achieve a range over seven orders of magnitude. The urine samples were subject to protein precipitation with acetonitrile prior to analysis by UPLC-IMS-MS.
The samples were chromatographed on an ACQUITY UPLC BEH Amide, 1.7 µm, 2.1 x 100 mm Column (p/n: 186004849), operated under HILIC gradient conditions over 10 min. The mobile phases consisted of 10 mM ammonium formate and formic acid in 95:5 water:acetonitrile (mobile phase A) and 5:95 water:acetonitrile (mobile phase B). The column effluent was monitored using a Vion IMS QTof operating in HDMSE positive or negative mode with a collision energy ramp from 20–30 eV and a mass range of m/z 50–1200.
A typical calibration line obtained for the sulfadimethoxine and verapamil are shown below in Figure 1 and reserpine and sulfagaunidine in Figure 2. The data acquired shows linear response over four orders of magnitude for all the spiked compounds.

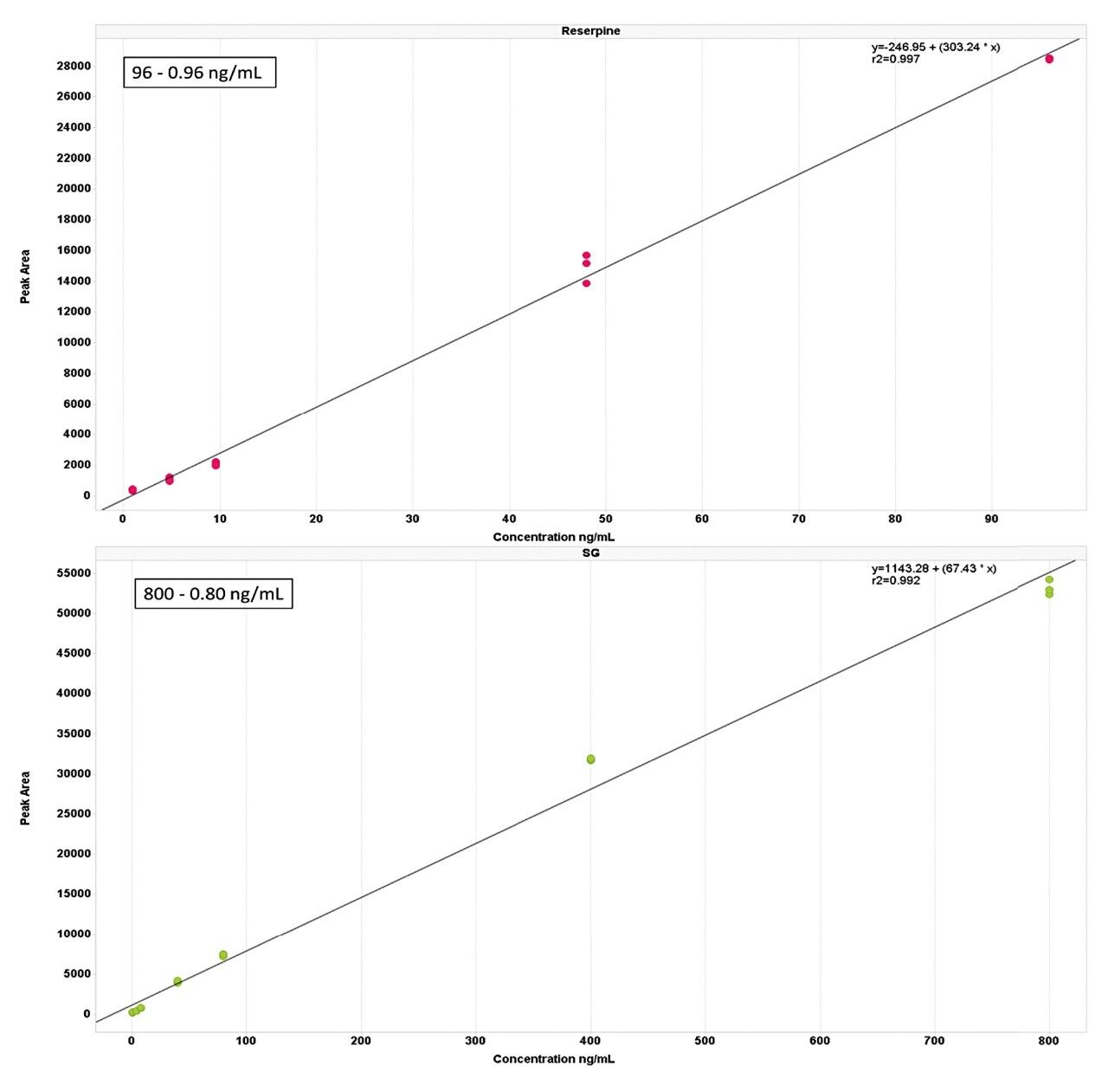
Metabolic profiling provides a unique insight into biochemical changes as a result of disease or toxicity. The biological variation that can be seen when comparing diseased subjects to those of healthy controls produces a broad response from metabolites often to the extremes of instrument capabilities. The UPLC-MS/MS system showed a linear dynamic range over three orders of magnitude for small molecules in urine, making the system ideal for the semi-quantitative measurement and identification of metabolites in biological fluids.
720006643, September 2019