For forensic toxicology use only.
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the development of a single sample preparation method, using Waters Oasis MCX 96-well µElution Plate, for the analysis of a mixed drug panel containing Tetrahydrocannabinol (THC) that can be applied to different oral fluid collection devices.
Inclusion of THC into an oral fluid mixed analyte panel using a single sample preparation method
The requirement to analyze drugs at low levels in oral fluid has become an important requirement for many forensic laboratories around the world. Many different collection devices are commercially available which offer a simple way of collecting oral fluid samples in a non-invasive, yet supervised, manner. These devices provide the laboratory with either neat oral fluid or oral fluid that has been diluted in a variety of different preservative buffers. The lack of standardization for collection devices highlights the need for a sample preparation strategy suitable for all analytes that can be used with all the commonly available devices. The most commonly detected drug in these schemes is THC, however many other drug classes such as opiates, opioids, amphetamines, and benzodiazepines must also be measured, and as sample volume can be limited, this has to be performed using a single sample preparation method. Oasis MCX µElution offers the ability to extract all of these analytes from limited volumes of this complex matrix with sufficient efficiency to meet current guidelines such as those applied by the European Workplace Drug Testing Society (EWDTS).1
The oral fluid collection devices tested in this study were the Salivette saliva collection device from Sarstedt, the Quantisal Oral Fluid Collection Device from Immunalysis, and the Saliva Collection System from Greiner Bio-One. Control oral fluid samples were collected as per the manufacturer’s instructions and spiked with a mixture of 27 illicit or prescription substances commonly measured in oral fluid.
The analytes were extracted from the matrix using a simple Oasis MCX 96-well µElution Plate (P/N 186001830BA) protocol, based on previously reported methods.2,3 The volume of sample loaded onto the plate for each collection device equated to the same volume (<75 µL) of neat oral fluid. The data was collected using a dual transition MRM method (quantifier and qualifier ions for each analyte) and processed using the TargetLynx Application Manager.
The chemical properties of THC are very different to the other analytes in the panel, and as such, THC requires a different chromatographic gradient to be able to meet the 2 ng/mL cut-off for confirmation tests recommended by the EWDTS guidelines for oral fluid analysis, as seen in Figure 1.
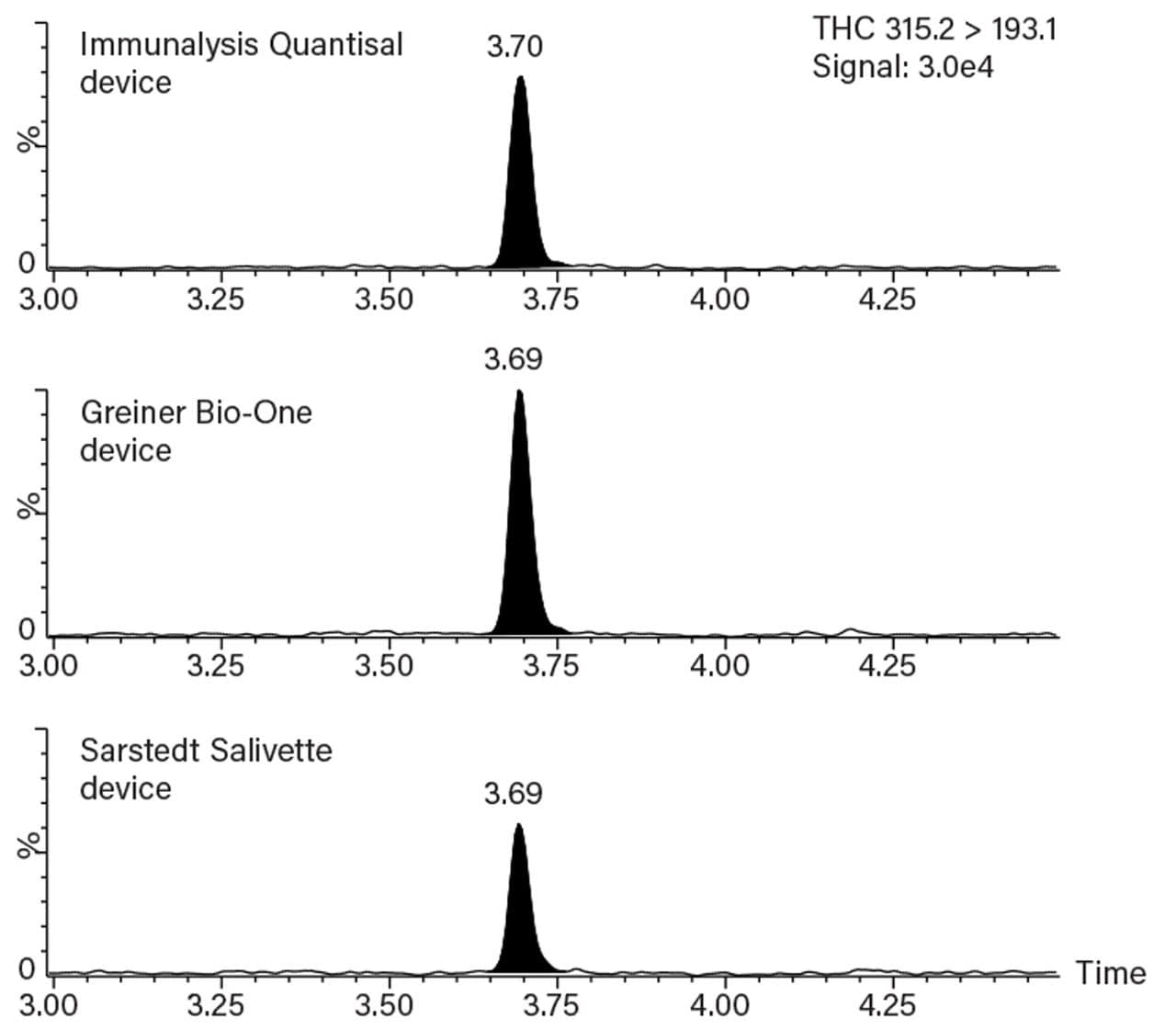
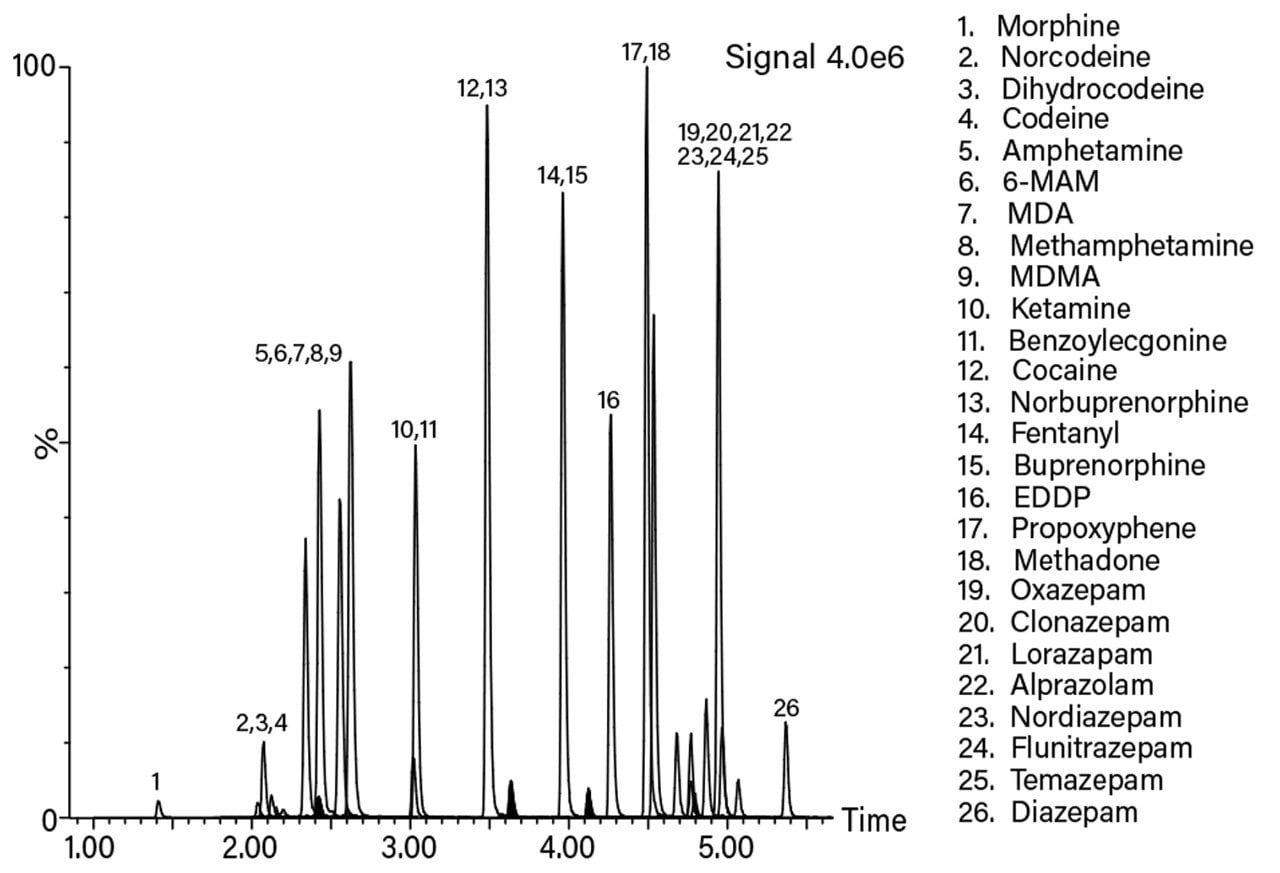
The eluant from the Oasis MCX µElution Plate was split into two equal aliquots in a 96-well Sample Collection Plate; 700 µL Round well (P/N 186005837). Following evaporation under nitrogen the separate aliquots were reconstituted in one of two alternative solvents. One aliquot was reconstituted in 50% acetonitrile and analyzed specifically for THC; the other aliquot was reconstituted in 5% acetonitrile containing 0.1% blank human plasma and used to determine all the other compounds. Figure 2 shows the chromatographic separation of all the analytes (except THC) scaled to the most intense.
To avoid the need for column switching, the two analytical methods were run on the same ACQUITY UPLC BEH C18 Column (P/N 186002352) using the same mobile phases, allowing for the methods to be run consecutively.
The high sensitivity Xevo TQ-S micro mass spectrometer in conjunction with the ACQUITY UPLC I-Class System (FTN) is ideally suited to this application as the analyte concentrations in oral fluid can be relatively low in comparison to other biological matrices.
The presence of multiple chemical classes with very different chemical properties in a drug panel, in combination with the limited sample volume that is often provided by oral fluid collection devices, creates a very challenging application. The use of a single Oasis MCX µElution sample preparation method and two UPLC-MS/MS methods allow for the analysis of a mixed drug panel, which includes THC, in less than 15 minutes and at concentrations which meet current guidelines such as those applied by the EWDTS. This sample preparation method can be easily automated to increase sample throughput.
720006081, August 2017