
In this application note, Atmospheric Pressure Gas Chromatography (APGC), coupled with a highly sensitive tandem quadrupole mass spectrometer, Xevo TQ-S, is demonstrated as a sensitive and robust option for confirmatory analysis of PCDDs and PCDFs by GC-MS/MS in compliance with 589/2014/EU.
The term dioxins is commonly used in reference to polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs). These are a group of chemically related compounds, known to be toxic and persist as ubiquitous pollutants. Therefore, dioxins are restricted internationally under the Stockholm Convention,1 along with other nationally enforced regulations. Furthermore, the World Health Organization (WHO) has conducted human-based risk assessments, setting Toxic Equivalent Factors (TEFs) for these compounds.2 For example, congener 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) has been deemed the most toxic, and has been assigned a TEF of one. In accordance with Regulation 1881/2006/EC dioxin levels must be reported as Toxic Equivalences (TEQ), where the determined concentration is multiplied by the TEF, and individual values are summed.
Given their persistence and toxicity, analytical methodology is required to ensure food safety and consumer protection. Due to the legislative requirements in Europe and America the gold standard for dioxin analysis uses gas chromatography coupled with high resolution mass spectrometry (GC-HRMS), which has been traditionally operated by electron impact (EI). Much of the criteria for the analytical methodology is based upon the most toxic dioxin, 2,3,7,8-TCDD. Following recent technological advances, and after a detailed evaluation, the European Commission has legislated that tandem quadrupole (GC-MS/MS) may be used as a confirmatory method.3 Regulation 589/2014/EU sets the performance criteria related to the specificity, sensitivity, confirmatory ions, resolution and calibration for the confirmation of dioxins in food and feed. This regulation is enforced in conjunction with existing requirements.4-6
In this application note, Waters Atmospheric Pressure Gas Chromatography (APGC), coupled with a highly sensitive tandem quadrupole mass spectrometer, Xevo TQ-S, is demonstrated as a sensitive and robust option for confirmatory analysis of PCDDs and PCDFs by GC-MS/MS in compliance with 589/2014/EU.
|
GC system: |
7890A |
|
Column: |
DB-5MS UI 60 m x 0.25 mm I.D. x film thickness 0.25 μm |
|
Injection: |
1 μL pulsed splitless mode |
|
Temp.: |
280 °C |
|
Pulse: |
450 kPa for 2 min purge 80 mL min-1 for 2 min |
|
Liner: |
Deactivated single gooseneck splitless liner (4 mm) |
|
Oven program: |
140 °C (2 min hold); 50 °C min-1 to 200 °C; 4 °C min-1 to 260 °C; 6.5 °C min-1 to 300 °C (10 min hold) |
|
Transfer line temp.: |
350 °C |
|
Carrier gas flow: |
1.5 mL. min-1 (helium) |
|
Make-up gas: |
300 mL min-1 (nitrogen) |
|
MS system: |
Xevo TQ-S |
|
Ionization: |
API+ by charge transfer |
|
Corona pin: |
2 μA |
|
Cone voltage: |
30 V |
|
Source temp.: |
150 °C |
|
Cone gas flow: |
200 L.h-1 |
|
Auxillary gas: |
275 L.h-1 (nitrogen) |
|
Collision gas: |
5 x 103 mbar (argon) |
|
Acquisition: |
Multiple Reaction Monitoring (MRM) mode, shown in Table 1 |
MassLynx Software with TargetLynx Application Manager
Standards EPA-1613 CSL to CS5 were used for calibration curves, containing both native and 13C labeled PCDD, PCDF, and TCDD compounds.
For sample preparation the following standards were used: EPA-1613 PAR, EPA-1613 LCS and TF-TCDD-MXB, along with 13C-labeled EPA-1613 ISS PCDD and PCDF congeners. All standards were purchased from Wellington Laboratories (Ontario, Canada). A further dilution of the CSL standard was made in nonane to give a 10 fg.μL-1 standard.
Sample preparation was completed in accordance with standard methods in accordance with Commission Regulations 252/2012/EC and 152/2009/EC.3-6
The results obtained for PCDDs and PCDFs by Xevo TQ-S with APGC will be discussed relative to the analytical requirements set out in Regulation 589/2014/EU. Some of the criteria are highlighted here.
Regulation 589/2014/EU requires that the resolution of each quadrupole in MS/MS analysis be set equal to, or better than one unit mass. This ensures sufficient selectivity in differentiating between the analytes of interest and interfering compounds. The default unit mass resolving power was retained on the Xevo TQ-S instrument.
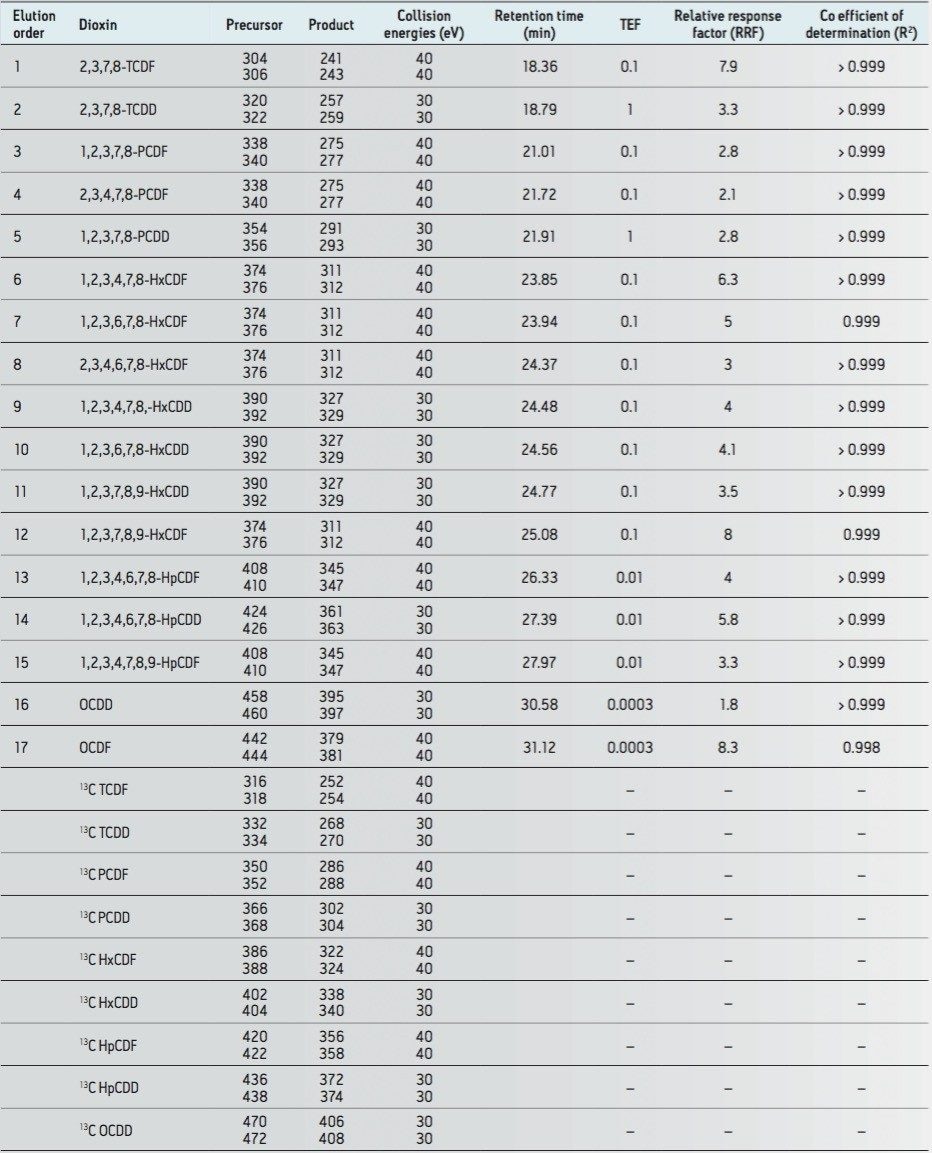
All data for labeled and unlabeled standards were acquired in MRM mode, providing excellent selectivity for two transitions per compound. These transitions are shown in Table 1, where two specific precursor ions are monitored, each with a specific corresponding product ion. This complies with paragraph 6.5 in Annex III of Regulation 589/2014/EU.
The excellent selectivity and specificity afforded by APGC with Xevo TQ-S is shown in Figure 1, where an overlay of all PCDD and PCDF congeners is provided. This data supports the requirements of Paragraph 5.2 (Annex III), requiring high selectivity in order to differentiate between the 17 congeners of 2,3,7,8-substituted PCDD/Fs.
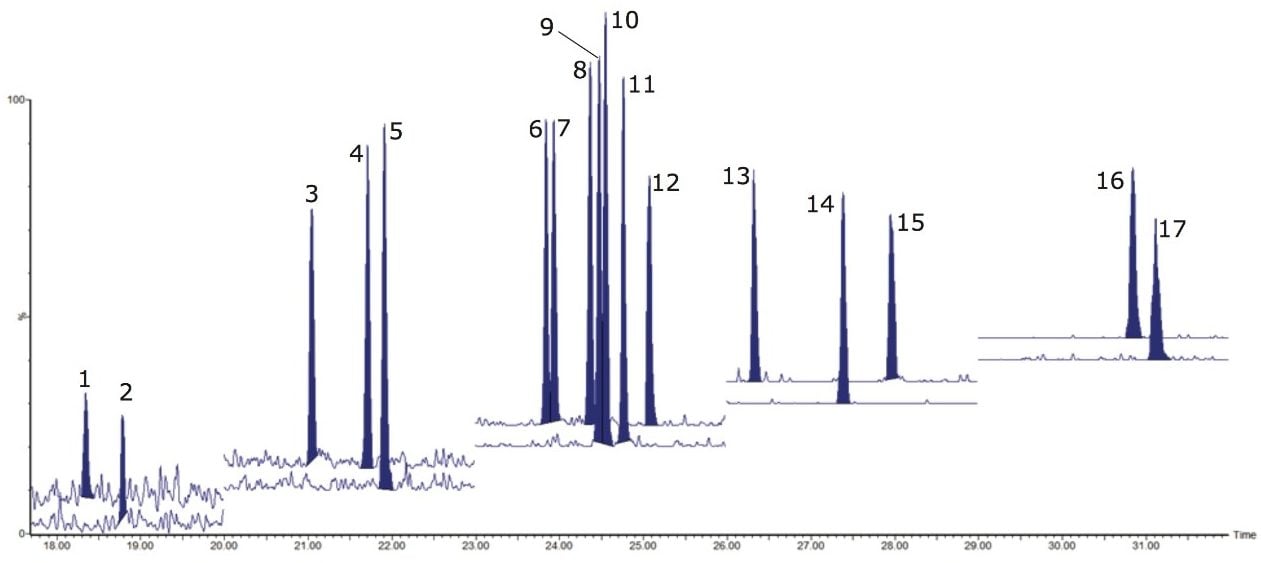
The use of MRM mode provides increased selectivity from co-extractives or interfering compounds, as shown in Figure 2. This was demonstrated during repeat analysis of blank extracts, where interferences were negligible. Carryover from sample to sample is always a concern when targeting these ubiquitous compounds at low levels. Using methodology previously reported by Ladak, et al.,8 a significant reduction in carryover of 20% was achieved, compared with the original methodology, thus allowing for the accurate quantification of dioxin analytes at trace level.
A confirmatory method must provide sufficient sensitivity to allow for control of food and feed at maximum/action levels. Therefore, Regulation 589/2014/EU proposes that the limits of quantification (LOQs) should be approximately one-fifth of the maximum limit for all PCDD and PCDF analytes, where PCDDs/Fs should be detectable in the upper femtogram range (10-15 g). Traditionally in HRMS methods, the 2,3,7,8- TCDD is monitored at 100 fg, ensuring that the signal-to-noise (S/N) is >100.
In this study, signal-to-noise ratios exceeded this traditional specification. Therefore, dilutions were made on the 2,3,7,8- TCDD standard. As shown in Figure 2B, excellent sensitivity was achieved for 10 fg.µL-1 (peak to peak S/N= 49) in compliance with Regulation 589/2014/EU.
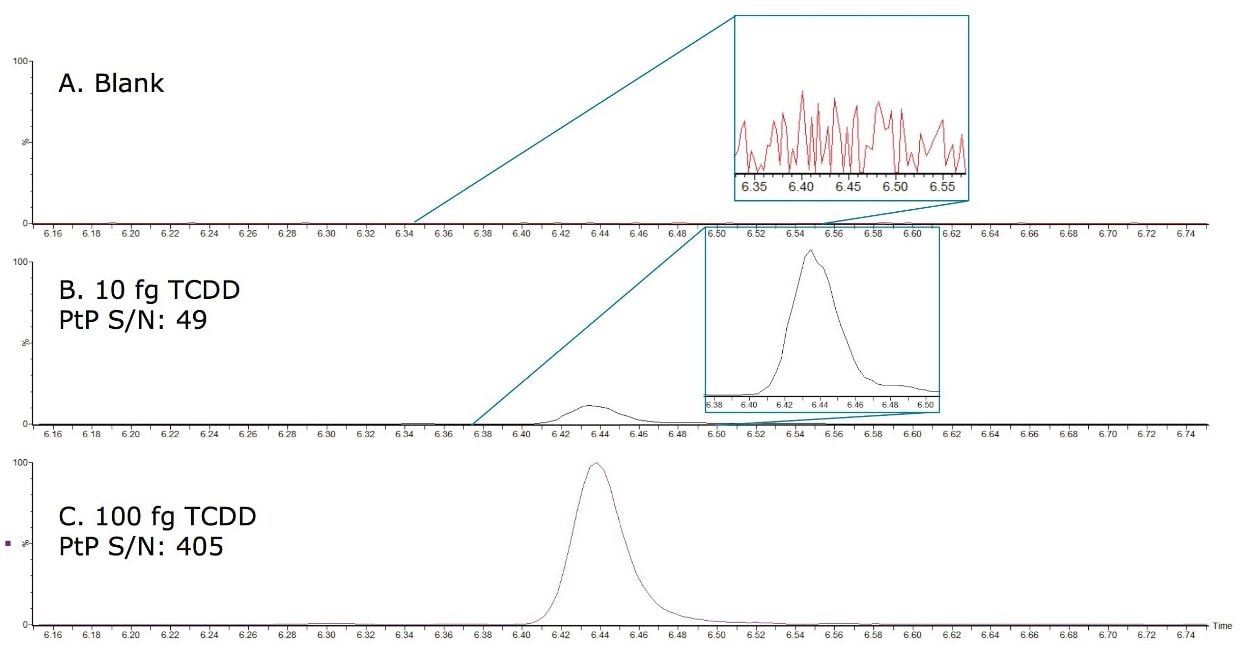
A primary requirement for many confirmatory methods using MS/MS is good agreement of the ion intensity ratios relative to the calculated values of the calibration standards. This is now implemented for dioxin analysis by MS/MS with a maximum permitted tolerance of 15%, where the two isotopic precursors ([M+• 35Cl] and [M+• 37Cl]) are made relative to their product ion (predominant loss of [CO35Cl] and [CO37Cl] respectively). Utilizing the automated facility in TargetLynx Application Manager, the measured ion ratios for the calibration standards were averaged and all of subsequent samples were compared to this. Activating the flagging system, any ion ratios exceeding the permitted tolerance of 15% were highlighted for further investigation.
In this study, all relative ion intensities were compliant with the permitted tolerance of 15%, providing confirmation of analyte detection in compliance with Regulation 589/2014/EU.
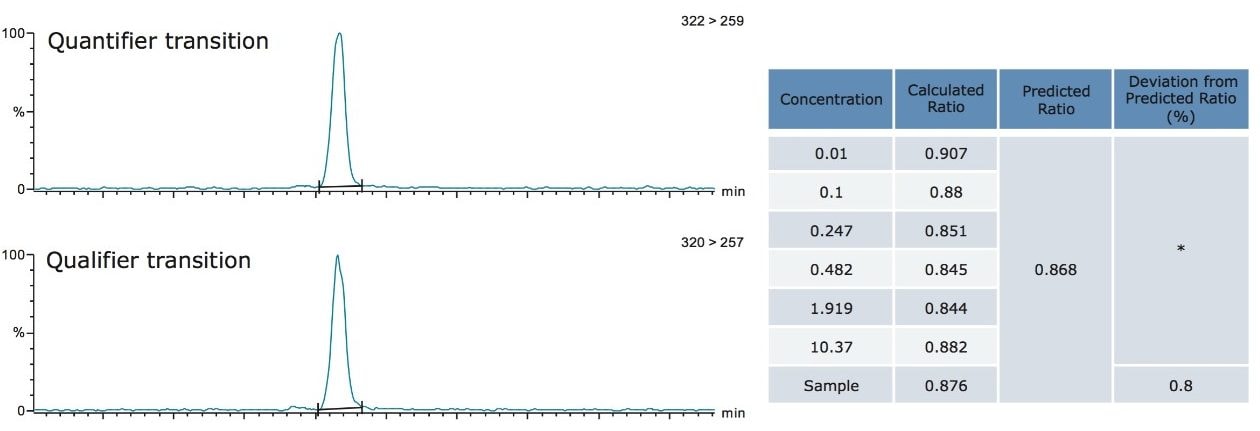
Figure 3. Quantification and qualification ions for TCDD showing calculated intensity ratio for the sample is compliant with ±15% tolerance enforced by Regulation 589/2014/EU.
*Predicted intensity ratio is an average of calculated ratios for all calibration standards.
Calibration curves are a basic necessity for accurate quantification of analytes. Regulation 589/2014/EU requires calibration curves for each analyte of interest over a range that satisfies the maximum levels of that compound. Excellent linearity was obtained for all PCDD and PCDF analytes over an appropriate working range of 0.01 to 40 pg.µL-1 (on column) containing seven calibration standards. An example of this linearity is shown in Figure 4 for TCDD.
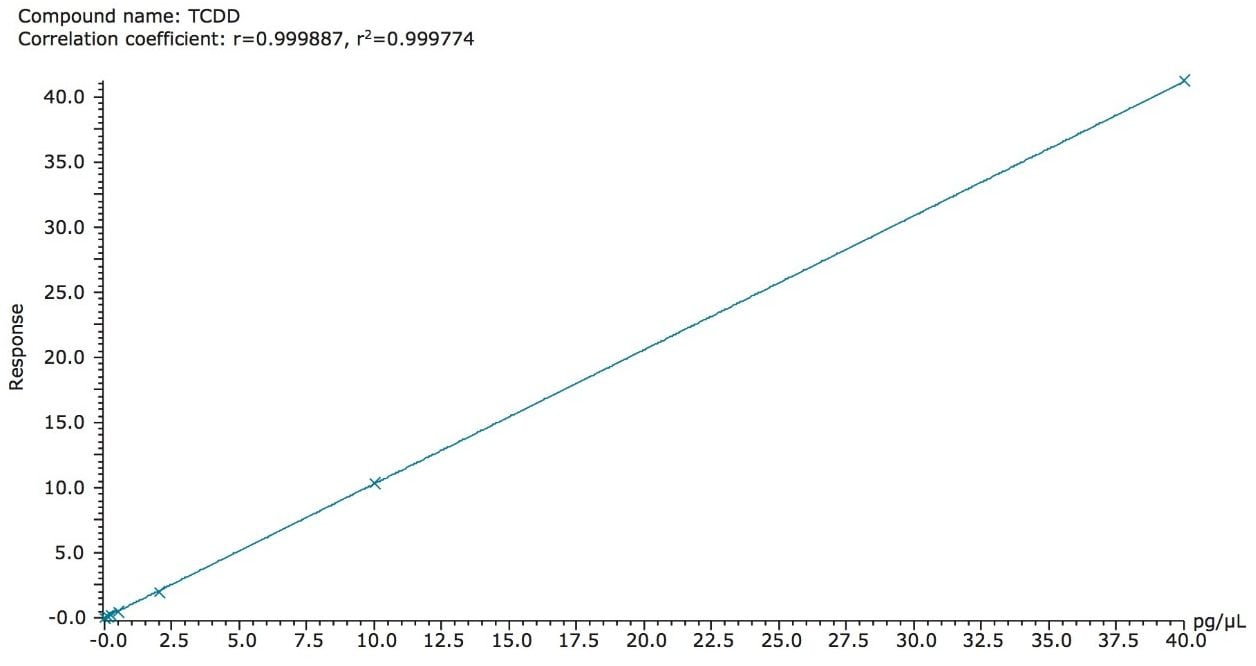
Further linearity results are shown in Table 1, where co-efficient of determination (R2) values and relative response factors (RRFs) for each analyte are summarized. Excellent RRF (%RSD of calibration standards from known concentration) values were achieved for all analytes, in compliance with the requirements set out in Regulation 589/2014/EU (<30% for lowest calibration standard), and EPA method (<15% for all subsequent calibration standards).
Therefore, the Xevo TQ-S Mass Spectrometer coupled with APGC is deemed compliant with Regulation 589/2014/EU and can be used as a confirmatory method for the analysis of PCDDs and PCDFs in foods and feeds. Further analysis was conducted in order to compare traditional HRMS results to those obtained by the new APGC-MS/MS method. Various food samples were prepared as referenced previously and analyzed on both GC-HRMS and APGC-MS/MS systems. Excellent agreement was observed between the two analytical platforms, at concentrations that were well below the permitted limits (<0.1 pg.µL-1). This is shown in Figure 5 for mixed animal fat, where similar levels of dioxins were detected by both mass spectrometry systems.
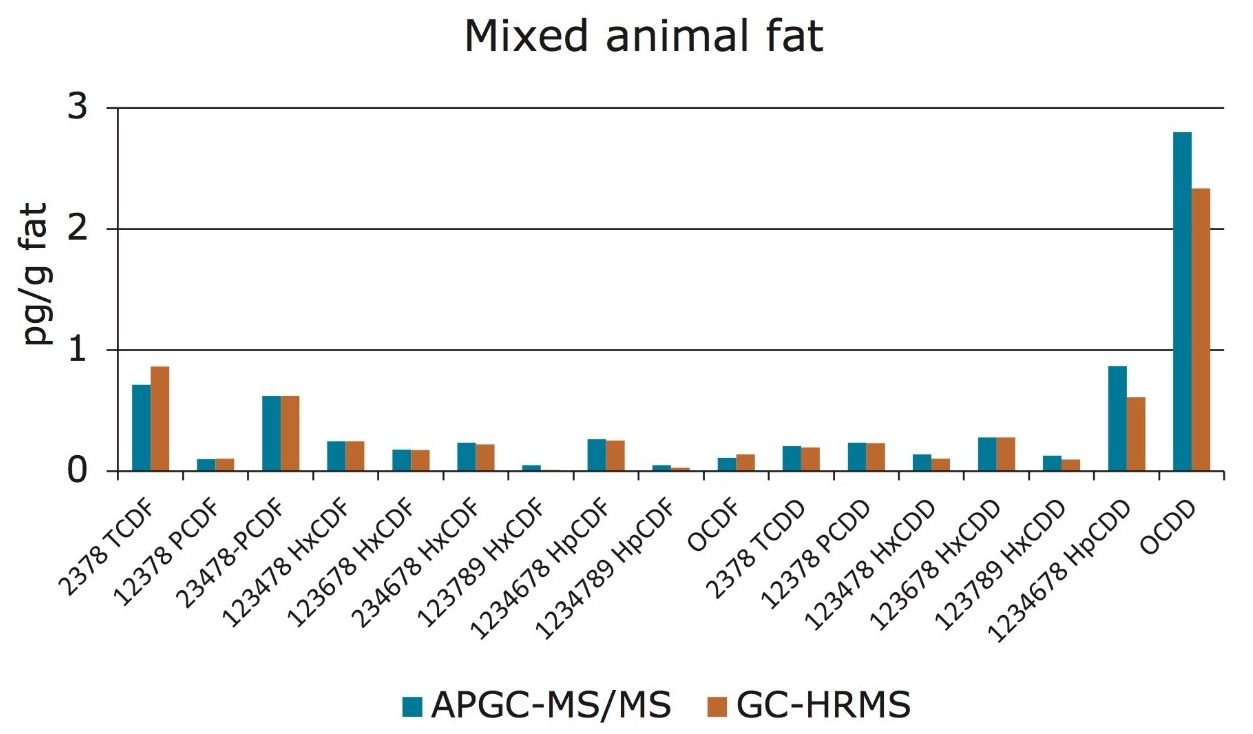
The excellent sensitivity afforded by the Xevo TQ-S allows for low injection volumes without compromising data quality. By injecting only 1 µL, the possibility of contamination of the liner, column, and/or instrument from matrix has been significantly reduced. This in turn improves the lifetime of GC consumables and reduces the need for frequent instrument maintenance.
Coupling atmospheric pressure gas chromatography (APGC) with Xevo TQ-S provides excellent sensitivity and selectivity required for the confirmation of PCDDs and PCDFs in food and feeds in accordance with recent changes to European Commission Regulation 589/2014/EU and associated analytical requirements.
The softer ionization associated with APGC has allows for highly selective and specific fragmentation of the PCDD/Fs congeners. Operating in MRM mode, at least two specific precursor to fragment transitions were monitored per analyte, as required by Regulation 589/2014/EU. Furthermore, good agreement was achieved for abundance ratios of these fragments, relative to the calibration standards. Excellent linearity (R2 >0.995) was achieved for all PCDD and PCDF analytes over a working range of 0.01 to 40 pg.µl-1. All relative response factors were compliant with the maximum tolerance set out in Regulation 589/2014/EU.
Xevo TQ-S with APGC has been demonstrated as a reliable, sensitive, and selective replacement to the traditional gold standard of GC-HRMS, requiring less system maintenance and expertise for optimum operation.
720005431, June 2015