
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates how to rapidly detect and quantify low levels of phenylbutazone in muscle tissue using ultra sensitive UPLC-MS/MS.
Low detection limits can be achieved using this ultra-sensitive UPLC-MS/MS method to ensure the detection of contaminated products.
The recent findings of undeclared horsemeat in beef products in Europe has prompted an urgent requirement for industry and the regulatory authorities to analyze meat and meat products for the presence of horse tissue and residues of the nonsteroidal anti-inflammatory drug (NSAID), phenylbutazone (PBZ). Phenylbutazone is a veterinary medicine permitted in some EU Member States which is used for pain relief and to reduce inflammation in non-food producing animals (dogs and horses). However, it is not permitted to be used in the treatment of animals destined for the human food chain. Any presence of the substance in food of animal origin therefore results from the illegal use of carcasses of treated horses.
For this work, a published method1 for the extraction of phenylbutazone from muscle tissue was used with minor modifications. Briefly, 2 g of homogenized meat was shaken with 10 mL of acetonitrile for 2 minutes. Following centrifugation the supernatant was collected and diluted with water. The diluted extract from this first stage was then subjected to solid phase extraction (SPE) using Waters Oasis HLB 60 mg. Phenylbutazone was eluted with methanol and subsequently diluted with water containing 10-mM ammonium formate pH 3.2. By dilution of the extract before loading and following SPE, the need for evaporation and reconstitution was eliminated. For analysis by LC-MS/MS, 100 μL of the diluted extract was injected.
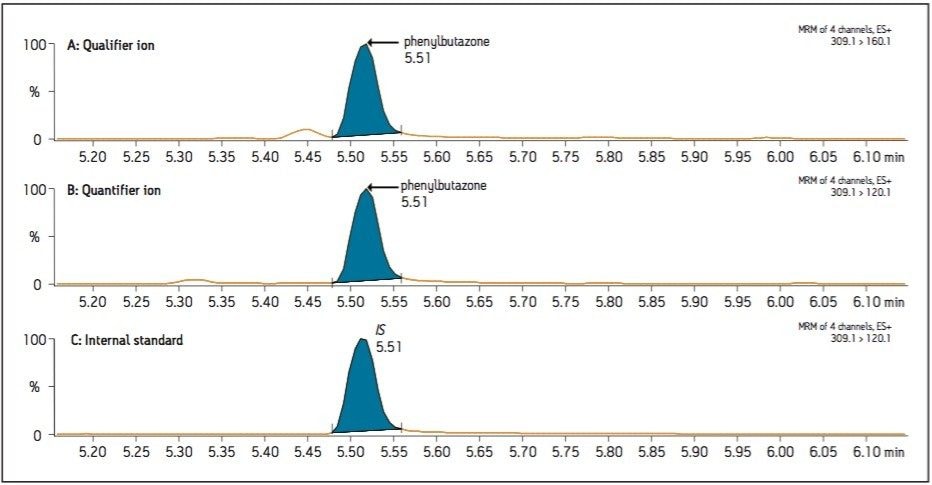
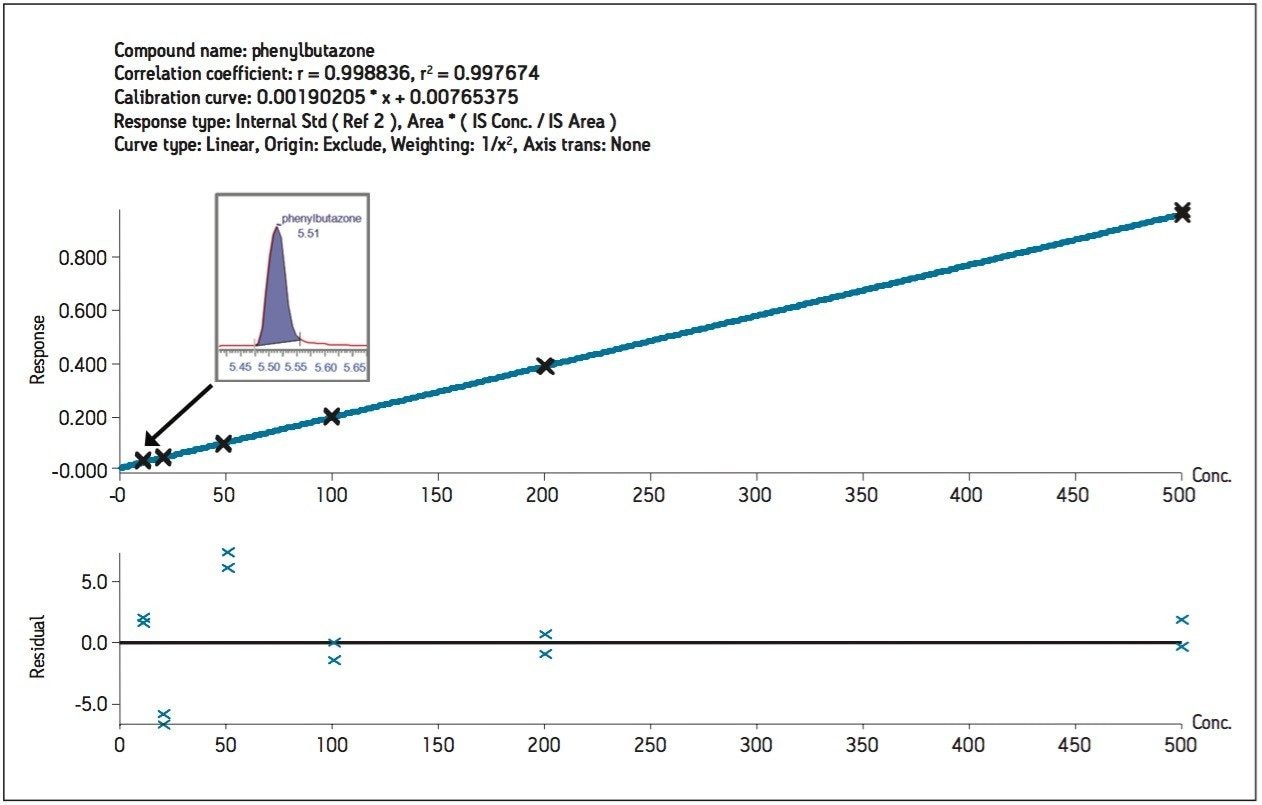
Two multiple reaction monitoring (MRM) transitions (quantification and confirmation) for phenylbutazone were selected and optimized. These results were added to the Quanpedia Database for future ease of use. For this application, the chromatography was performed on a 2.1 x 100 mm HSS T3 analytical column (1.7 μm) with a methanol/water, 10 mM ammonium formate (pH 3.2) 5-minute gradient. Figure 1 shows the MRM chromatograms resulting from the extraction of a 200 ng/kg spike of phenylbutazone. As shown in Figure 1, excellent signal-to-noise and peak shape were obtained for phenylbutazone and the internal standard (100 ng/kg). A recovery corrected calibration curve, shown in Figure 2, was spiked into matrix in duplicate and extracted to create a six point calibration curve. The calibration curve was calculated with a linear regression (1/x2 weighting) using phenylbutazone-diphenyl-13C12 as an internal standard. As can be seen from the data, the lowest calibration point (10 ng/kg) was easily detected and enables the analysis of phenylbutazone at 500 times lower than the EU recommended concentration (RC) of 5 μg/kg, with good linearity (R2 0.997) in the concentration range tested (10 to 500 ng/kg). This sensitivity is an important consideration in processed meat products where horsemeat may be combined with meat from other sources. In those cases, the levels of phenylbutazone may be well below the EU RC.
The ACQUITY UPLC I-Class coupled with the Xevo TQ-S enabled the analysis of phenylbutazone at 10 ng/kg in meat using Waters ACQUITY UPLC HSS T3 chemistry. The low detection limits that can be achieved using this ultra-sensitive UPLC-MS/MS ensure the detection of contaminated products, even when considering mixed and processed meat products.
720004668, April 2013