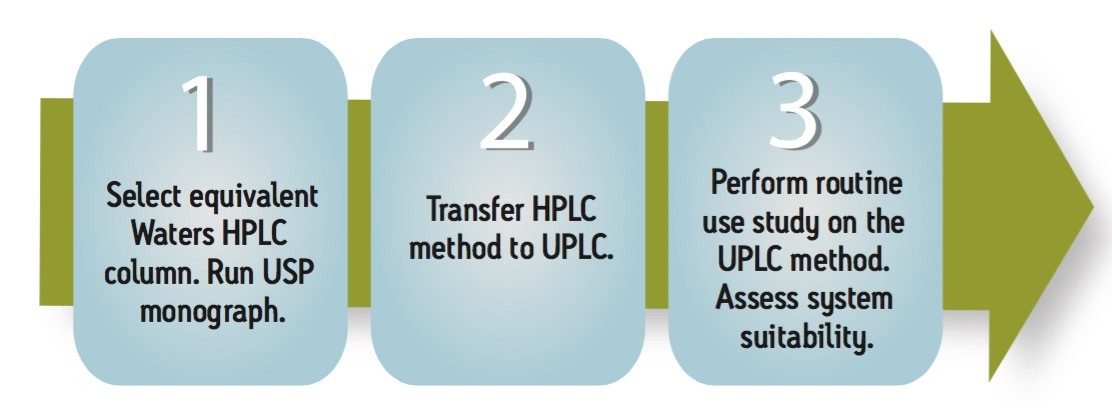
The simple workflow provides an approach for QC laboratories to transfer compendial methods from HPLC to UPLC. In this application note, the USP method for lamotrigine was successfully transferred to UPLC technology.
The pharmaceutical industry struggles with solving method transfer challenges. Implementation of UPLC increases overall profitability and as a result, pharmaceutical companies are transferring their legacy USP HPLC methodology to UPLC to increase efficiency within the QC environment. Therefore, simplified approaches supplemented with proven examples are essential to assisting their method translation goals with experience.
The compendial method for lamotrigine is a good representation of typical conditions described in other USP HPLC methodology. Lamotrigine is an anticonvulsant drug approved by the FDA and marketed by GSK as Lamictal primarily to treat seizures of specific diagnosis experienced by patients with epilepsy. For the treatment of bipolar depression, one-third of surveyed psychiatrists identify lamotrigine as the therapy with the greatest overall efficacy when compared to other currently available treatments.1 Since the patent expiration in 2008, a report published by Allbusiness.com titled “GlaxoSmithKline (GSK) Q1- 2010” identified competition from generic pharmaceuticals accounted for a 26% y-o-y decrease of Lamictal sales since 2008.2
The goal of transferring the USP Lamotrigine HPLC methodology to UPLC was achieved by following the workflow illustrated in figure 1 derived from the strategy described in a previous application note.3 A drug formulation analysis procedure was developed based on the drug substance methodology found in the USP for lamotrigine.4 Method feasibility was determined through routine use studies designed to assess applicability within quality control laboratories.
|
Buffer: |
20 mM potassium phosphate monobasic |
|
Mobile Phase: |
A: 150:1 buffer:triethylamine adjusted to pH 2.0 with phosphoric acid B: acetonitrile |
|
Detection: |
UV at 270 nm |
|
Column: |
XBridge C18 , 4.6 x 150 mm, 5 μm (USP designation: L1), part number 186003116 |
|
Needle Wash: |
95:5 water:acetonitrile |
|
Seal Wash: |
95:5 water:methanol |
|
Sample Diluent: |
0.1 M hydrochloric acid |
|
Flow Rate: |
1.0 mL/min |
|
Column Temp.: |
35 °C |
|
Injection Volume: |
10 μL |
|
Detection: |
UV at 270 nm |
|
Buffer: |
20 mM potassium phosphate monobasic |
|
Mobile Phase: |
A: 150:1 buffer:triethylamine adjusted to pH 2.0 with phosphoric acid B: acetonitrile |
|
Detection: |
UV at 270 nm |
|
Column: |
ACQUITY UPLC BEH C18, 2.1 x 50 mm, 1.7 μm, part number 186002350 |
|
Weak Wash: |
95:5 water:acetonitrile |
|
Strong Wash: |
50:50 acetonitrile:water |
|
Diluent: |
0.1 M hydrochloric acid |
|
Flow Rate: |
0.61 mL/min |
|
Column Temp.: |
40 °C |
|
Injection Volume: |
0.7 μL (partial loop using needle overfill mode) |
|
Data Management: |
Empower 2 CDS |
|
Tailing Factor for Lamotrigine: |
NMT 1.5 |
|
Area %RSD for five replicate injections: |
NMT 1.5% RSD |
The samples were prepared by transferring an appropriate pooled number of tablets to a 1 L volumetric flask to obtain a concentration equivalent to 1.0 mg/mL lamotrigine. Tablets were dissolved in 200 mL water and 800 mL methanol. This solution was mechanically shaken for 20 minutes followed by centrifugation at 4000 rpm for 20 minutes. Aliquots from the dissolved tablet sample solution were diluted with diluent to obtain a working sample concentration of 0.2 mg/mL.
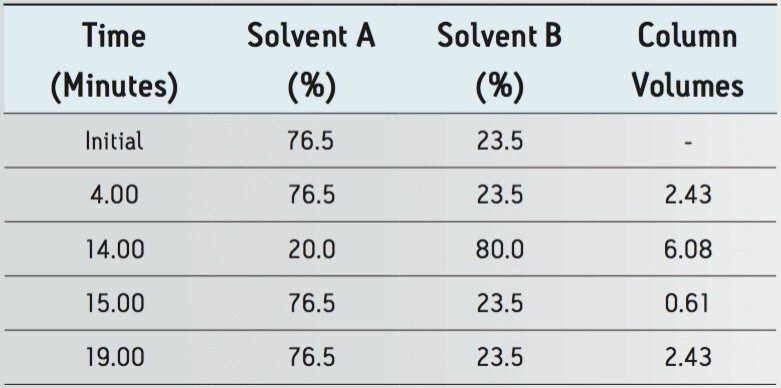
The USP HPLC method for lamotrigine and related compounds was validated using a Hypersil BDS C18 4.6 x 150 mm, 5 μm column (USP designation: L1), which does not have a commercially available sub-2 μm column configuration. The Waters Reversed-Phase Column Selectivity Chart identified XBridge C18 as an equivalent L1 stationary phase scalable to sub-2 μm particles. The HPLC methodology was performed as written on the XBridge C18 column (Figure 2a) meeting the system suitability requirements stated in the USP.
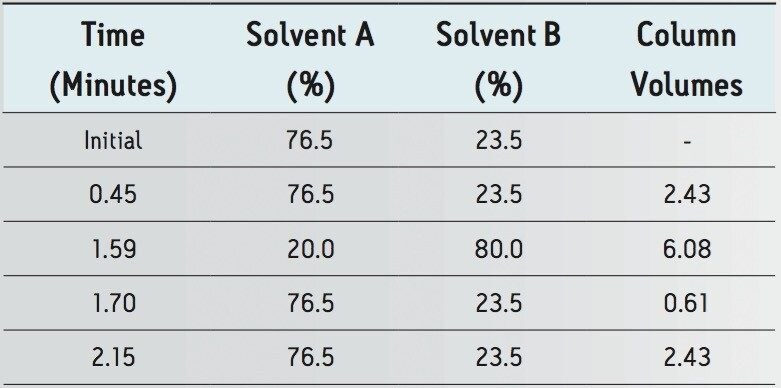
The HPLC method was transferred to an ACQUITY UPLC BEH C18 column using the Waters ACQUITY UPLC Column Calculator. The calculator specified two options for transferring to UPLC methodology: “accounting for particle size” and “disregarding particle size”. The conditions indicated for the option “disregarding particle size” is a direct geometric scaling of the chromatography. The “accounting for particle size” conditions scales the flow rate to the optimum potential of the particle size while maintaining the gradient segment column volumes providing increases in throughput without sacrifices in efficiency. For the purposes of this application, the method transfer was performed using the optimal method conditions which account for particle size to realize the full potential of savings when transferring to UPLC. The resulting chromatograms compared in Figure 2 show that the selectivity and resolution was maintained.
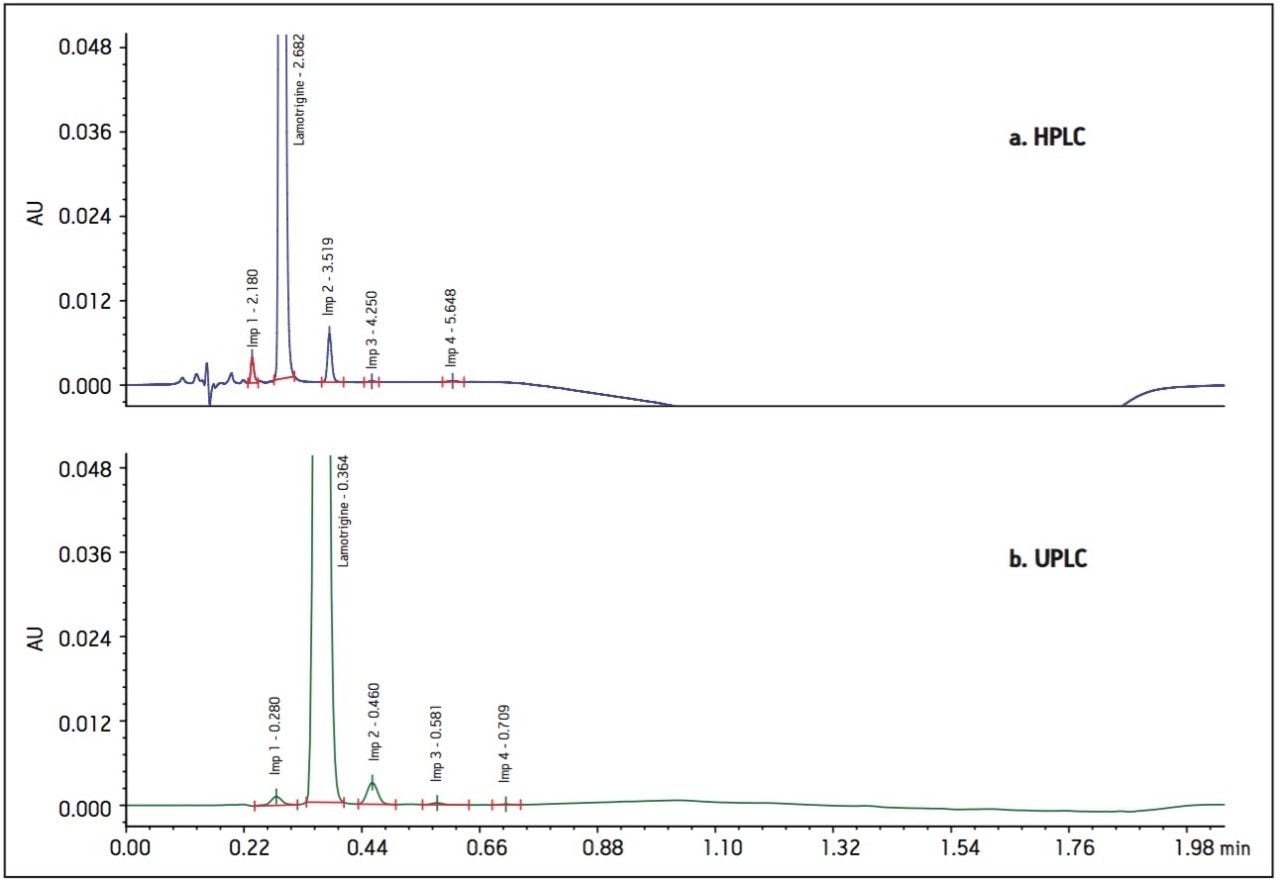
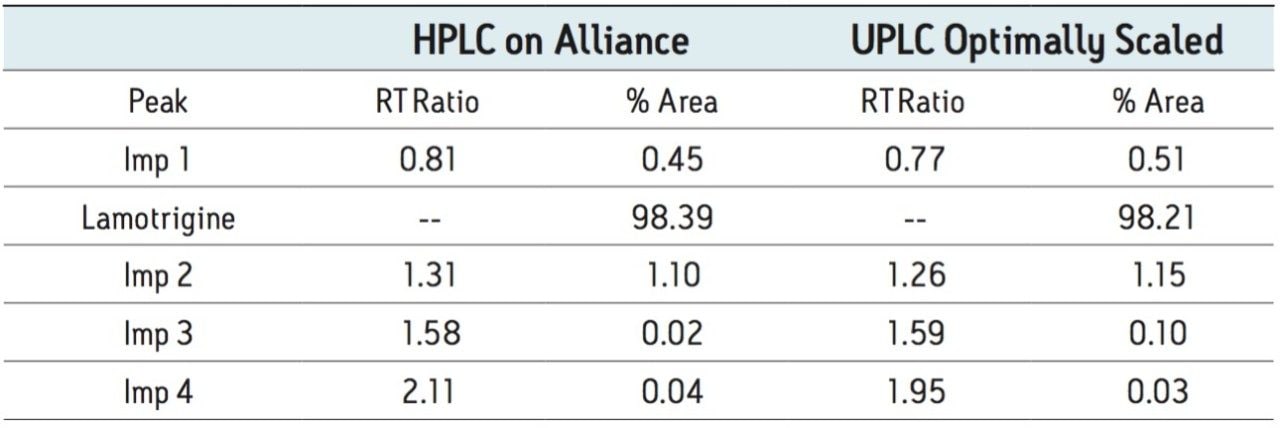
The methodologies were evaluated and compared to the HPLC results in Tables 1 and 2.
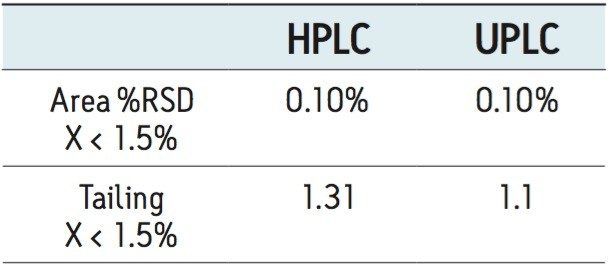
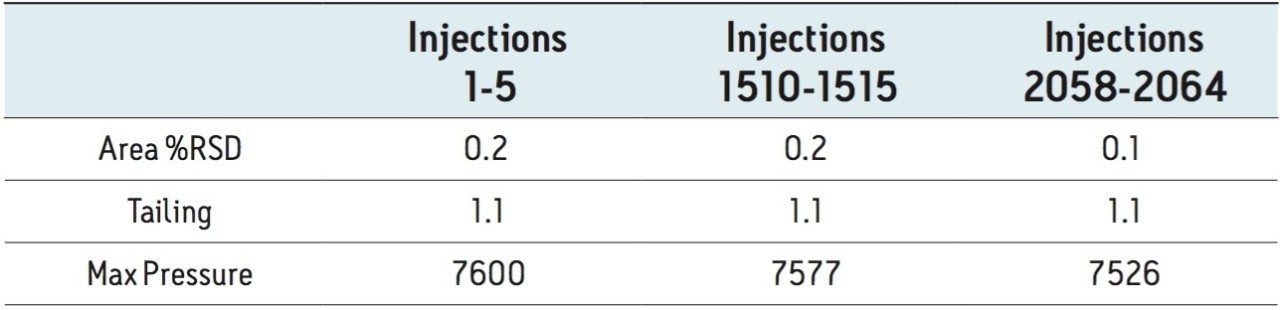
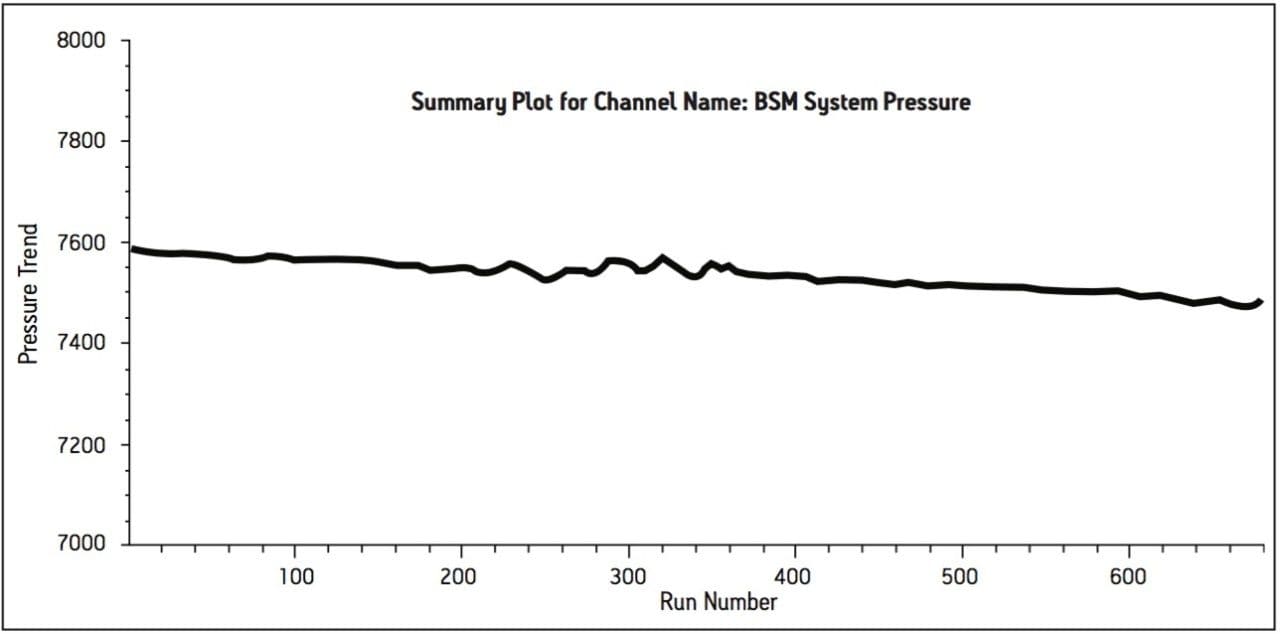
The UPLC method was evaluated in a routine-use study to more closely simulate what would be expected in a quality control laboratory. The USP system suitability criteria was assessed periodically throughout the study. The results successfully met the USP specifications for tailing and %RSD throughout the study and reported in Table 3. The routine-use study was completed at 3000 injections with no issues. Liquid chromatography methods analyzing drug formulation methodology can occasionally result in increases in system pressure due to improper sample preparation resulting from excipients collecting at the inlet frit of the column. System pressure was monitored and plotted for the duration of the routine-use study. The pressure remained stable throughout the study, as shown Figure 3.
The simple workflow provides an approach for QC laboratories to transfer compendial methods from HPLC to UPLC. The USP method for lamotrigine was successfully transferred to UPLC technology. The method was evaluated utilizing standards and tablet samples. Over 3000 injections were performed with no indication of reduction in UPLC methodology performance, as measured by the system suitability criteria specified in the USP method for lamotrigine. The UPLC method provides almost a 5-fold reduction in run time while maintaining the integrity of the system suitability criteria of the USP method. The UPLC method, as calculated for accounting for particle size, provides approximately 89% savings in run time and solvent consumption (2 minutes UPLC vs. 19 minutes HPLC), allowing quality control laboratories synergistic improvements that provide benefits in cost reductions.
720004132, April 2013