This application note demonstrates a rapid, simple isocratic method for the QC analysis of soft drink additives with minimal sample preparation. We use the quaternary ACQUITY UPLC H-Class System with UV detection and the Waters Beverage Analysis Kit, which includes pre-packaged acetate-buffered ethanol eluent to enable a separation of the analytes in less than seven minutes.
The soft drink market is an important worldwide business that generates profits for several major manufacturers. To ensure consistency of products and to satisfy quality control requirements, accurate quantification of additives is essential. Six additives that are commonly used are sodium benzoate and potassium sorbate as preservatives; acesulfame K; aspartame and saccharin for sweetness (diet beverages); and caffeine. Some or all of these compounds may be present depending on the formulation of a particular beverage.
In the manufacturing environment, producers desire simple, rapid analytical techniques with minimal sample preparation. Non-hazardous solvents are also important, both for the safety of laboratory personnel, and to keep disposal costs to a minimum. Isocratic methods are often preferred to keep the method simple, remove the re-equilibration step, and allow for the potential to recirculate mobile phase. Although mobile phase recirculation is not typically used in analytical laboratories, in the manufacturing environment, it is commonly employed to reduce costs and minimize the need for technical involvement.
Here we show the efficacy of the ACQUITY UPLC H-Class System with the Waters Beverage Analysis Kit, which includes the pre-packaged acetate-buffered ethanol eluent to effect a separation of the above analytes in less than seven minutes. The only sample preparation required is sonication (to remove the carbonation) and filtration.

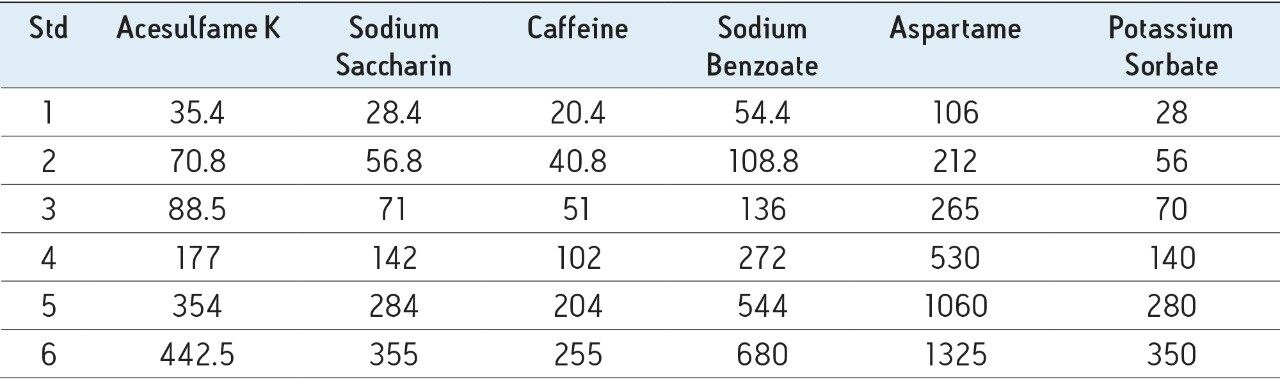
A stock standard was prepared by dissolving 0.177 g acesulfame K, 0.142 g sodium saccharin, 0.102 g caffeine, 0.272 g sodium benzoate, 0.530 g aspartame, and 0.140 g potassium sorbate in 10% aqueous ethanol, and diluting to 100 mL with same. From this, six dilutions were made in 10% aqueous ethanol to formulate a series of standards with concentrations listed in Table 1. These can be used for a multi-point calibration. When a single point calibration is preferred, Waters’ certified standard mix should be used.
This standard mix is available as part of the Waters Beverage Analysis Kit. A premixed standard containing five of the six analytes is supplied. Aspartame, which degrades over time in solution, is provided as a pre-weighed solid. All that is required for standard preparation is to pour the pre-dissolved liquid mix of standards into the bottle of solid aspartame, replace the cap, and shake the bottle until all the aspartame is dissolved. The final solution of mixed analytes can then be transferred to smaller standard vials for analysis.
Two diet colas (lime flavored and regular) and two diet fruit-flavored (lemon and orange) carbonated soft drinks were purchased at a local market. They were sonicated to remove carbonation and filtered through a 0.45 µm, 13 mm PVDF disc.
|
LC system: |
ACQUITY UPLC H-Class |
|
Runtime: |
7.0 min |
|
Column: |
ACQUITY UPLC BEH Phenyl 1.7 μm, 2.1 x 100 mm (PN 186002885) |
|
Column temp.: |
35 °C |
|
Mobile phase: |
Waters’ Beverage mobile phase (included in Kit PN 176002534) |
|
Flow rate: |
0.5 mL/min |
|
Injection volume: |
1 μL |
|
Detection: |
UV at 214 nm |
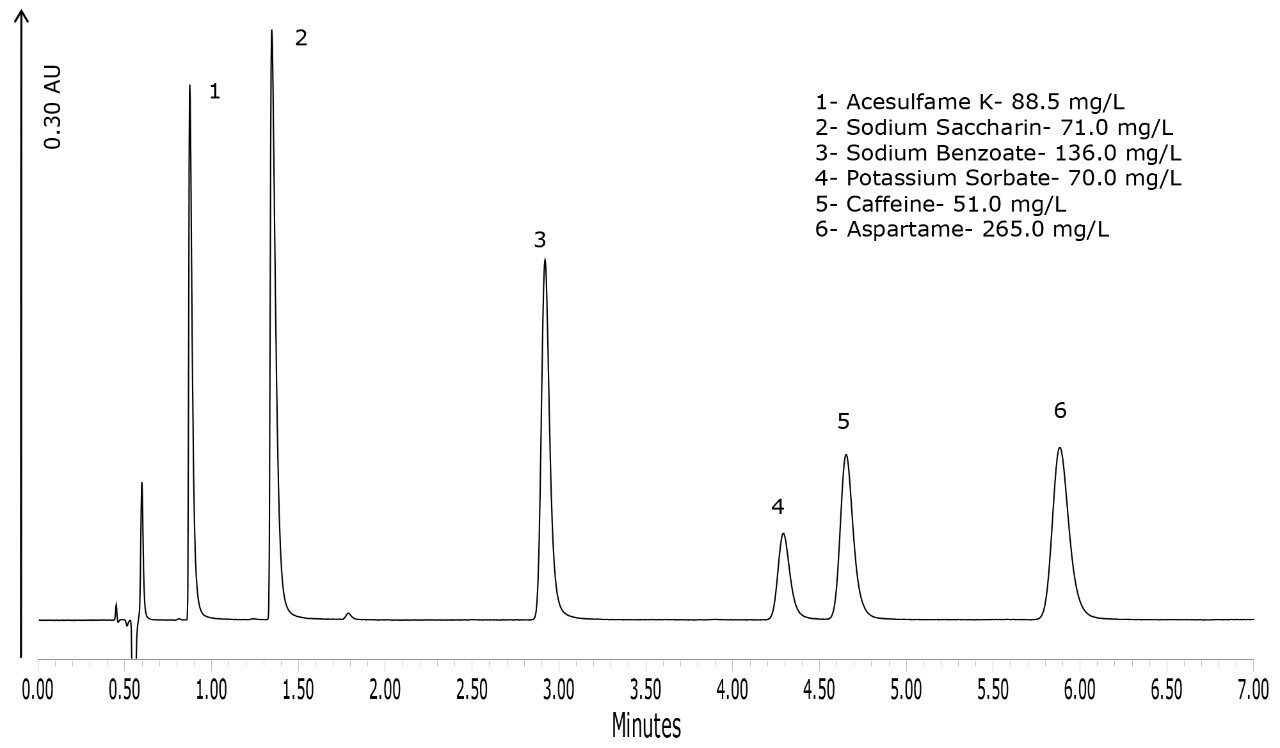
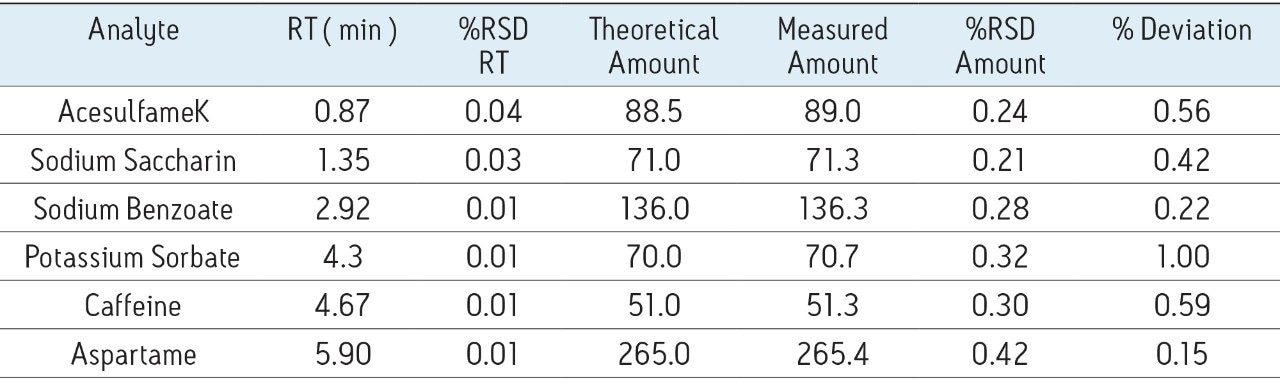
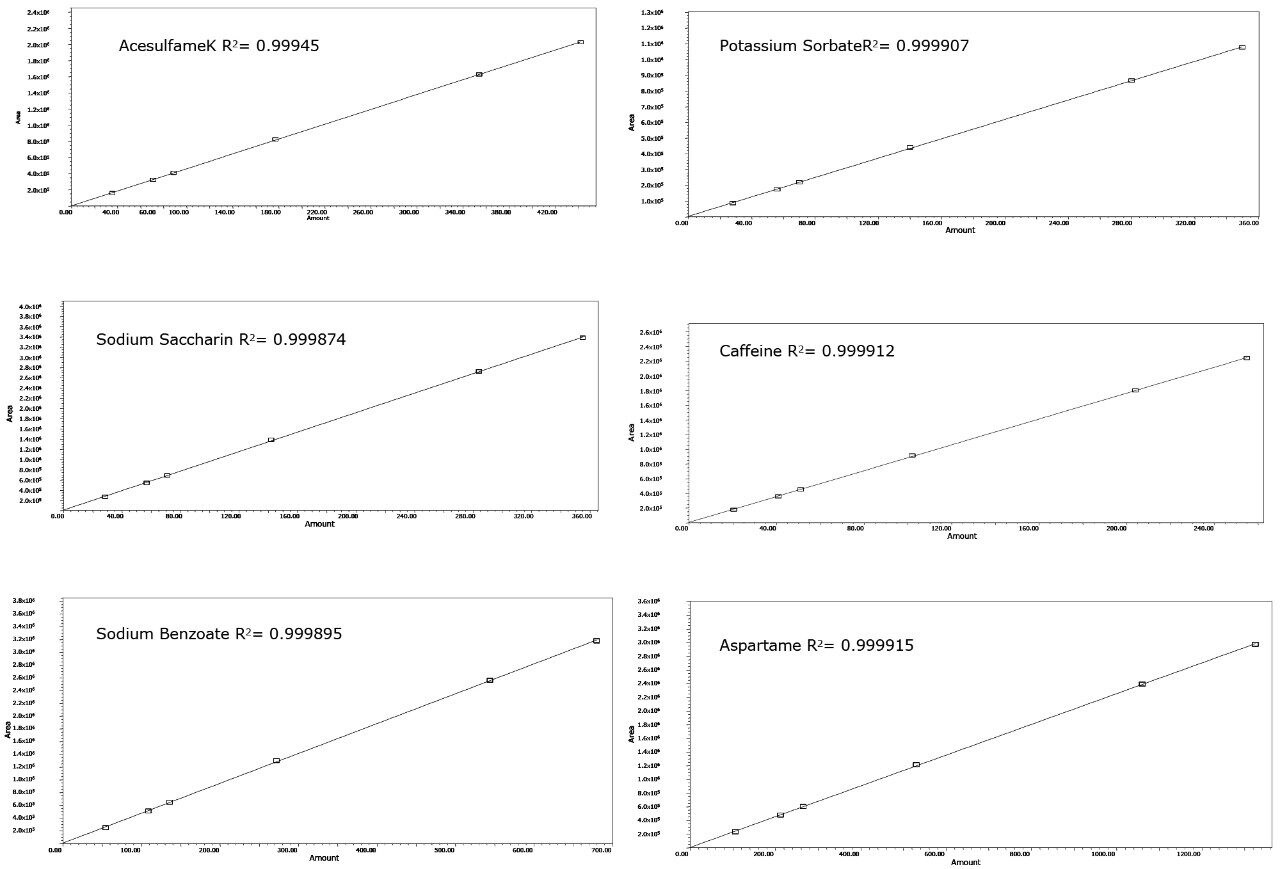
A typical separation of the six analytes of interest is shown in Figure 2. All analytes were baseline resolved and well separated. Seven injections of a QC standard at the same level as standard 3, shown in Table 1, were performed and the repeatability data is provided in Table 2. The % RSD for retention time was 0.04% or less for all analytes. When the data were quantified against the six-point calibration curve, the measured amount of each analyte was within 1% of the actual amount for all the analytes. When the data were quantified against standard three as a single point calibration, the measured amount of each analyte was also within 1% of the actual amount. The % RSD for the measured amount was 0.42% or less for all analytes. Calibration curves for the six analytes are shown in Figure 3. R2 values were greater than 0.999 for all compounds. Each standard was injected in triplicate.
To demonstrate the performance of the method for the types of products that would routinely be analyzed with this method, four products were purchased. The chromatograms of these products are shown in Figures 4 to 7. Figure 4 shows that the lime-flavored diet cola contained four of the six analytes: acesulfame K, potassium sorbate, caffeine, and aspartame. The plain diet cola shown in Figure 5, also contained four analytes but instead of potassium sorbate, sodium benzoate was used as the preservative. Sodium benzoate was also used in the diet-lemon and orange-flavored beverages, as shown in Figures 6 and 7, respectively. Of the four products, the orange-flavored beverage was the only product that did not contain caffeine.
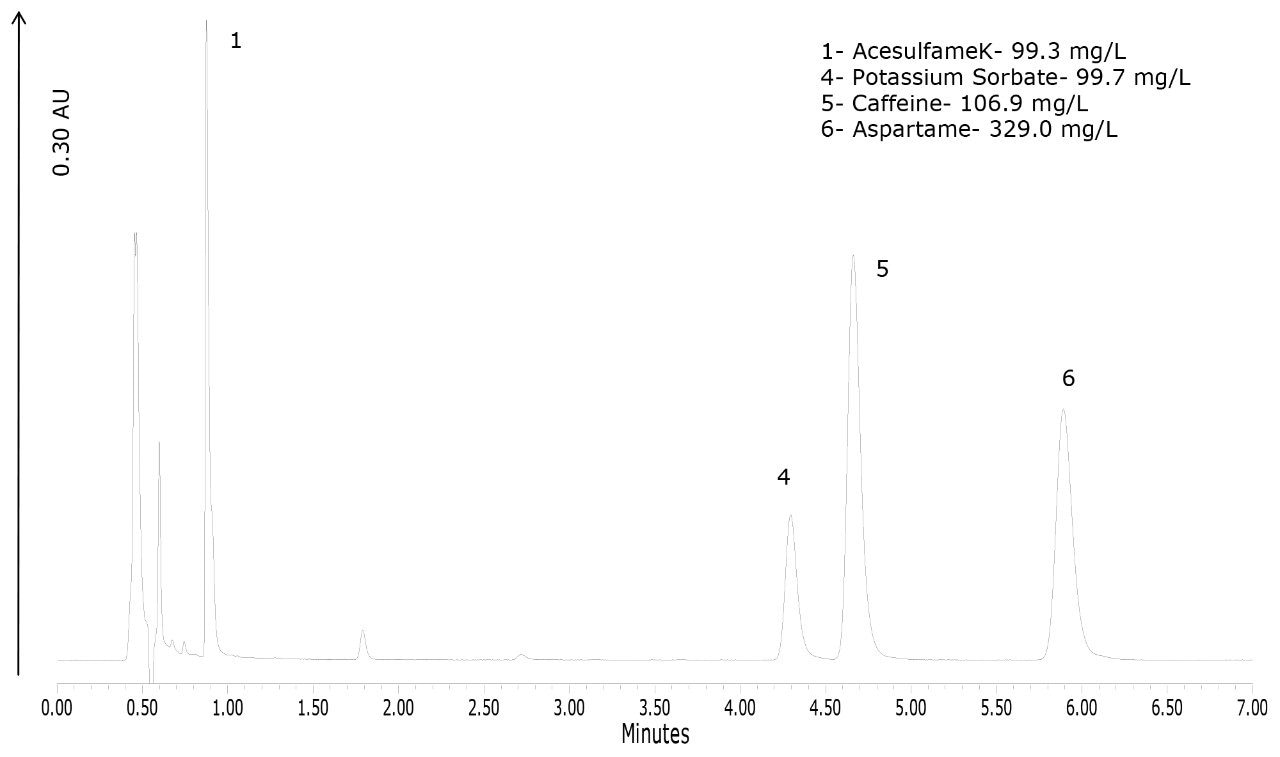
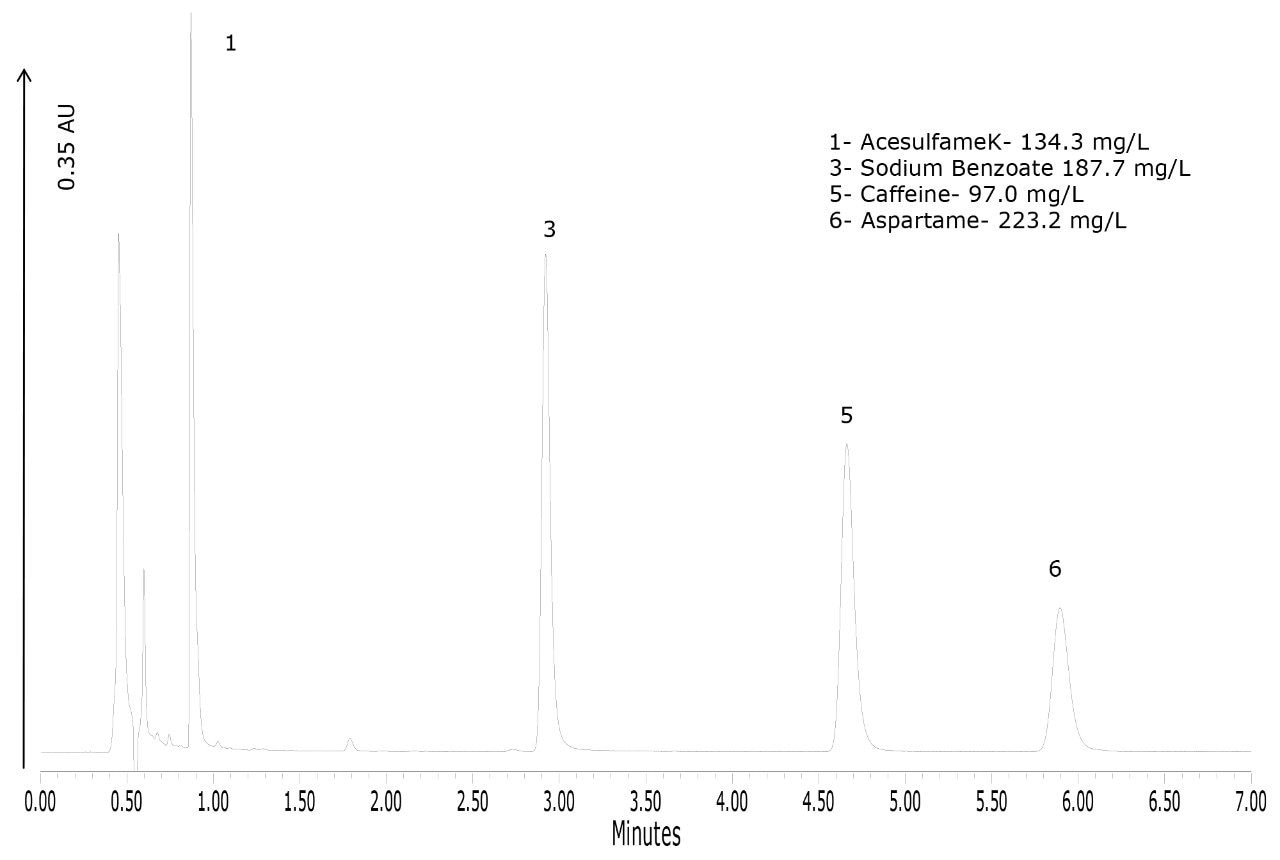
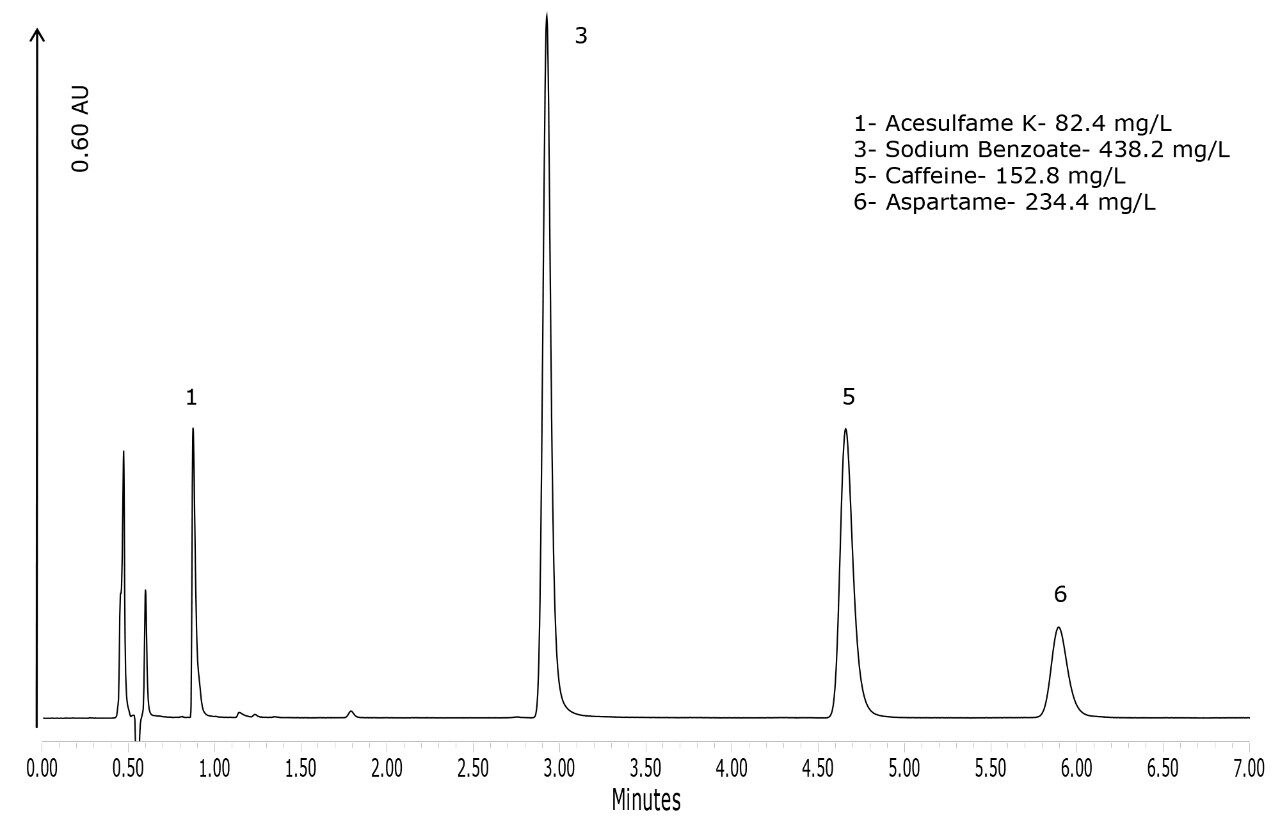
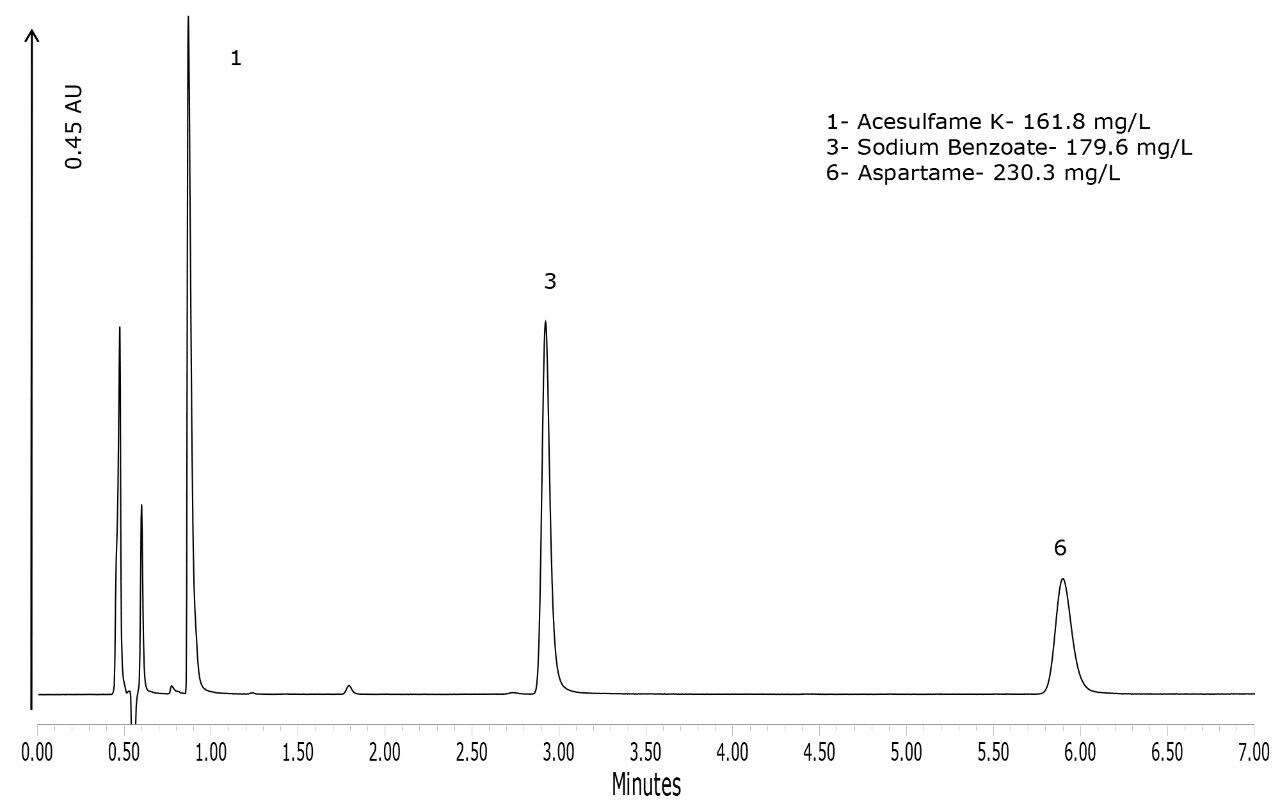
In terms of the product labeling, the observations listed above matched the ingredient list from each of the products. Caffeine is the only compound out of the six that has the concentration listed on the product label. Caffeine quantification was within 1.1% of the stated label claim for all three of the caffeinated beverages.
This application note has shown a rapid, simple method for the analysis of soft drink additives. Implementation of this procedure in a manufacturing environment has the capacity to improve overall workplace efficiency.
720004016, July 2011