LC-MS/MS using tandem quadrupole instruments is the technique of choice for quantitative analysis in drug metabolism studies, however it is typically less sensitive in full-scan than ion trap or time-of-flight instruments. The Xevo TQ-S tandem quadrupole mass spectrometer is equipped with novel StepWave transfer optics and large sampling orifice which dramatically increases both quantitative and qualitative assay sensitivity. Here we demonstrate the increase in full-scan sensitivity obtained with this new StepWave design using the metabolism of the common non-steroidal anti-inflammatory (NSAID) drug ibuprofen.
The new Xevo TQ-S tandem quadrupole MS system provides significant increase in full-scan sensitivity, allowing useful metabolite structural information to be obtained at much lower levels than previously achievable on a tandem quadrupole MS instrument.
The rapid detection of metabolites in drug discovery DMPK studies allows the project team to quickly evaluate and compare compounds with a similar core structure produced from a parallel synthesis experiment. The ability to obtain both qualitative and quantitative data in one simple analytical run improves laboratory productivity. This is of particular importance in an area where tens to hundreds of new candidate discovery compounds are analyzed per week. To address this productivity issue requires an instrument that is capable of acquiring both high sensitivity quantitative and qualitative data.
LC-MS/MS using tandem quadrupole instruments is the technique of choice for quantitative analysis in drug metabolism studies, however it is typically less sensitive in full-scan than ion trap or time-of-flight instruments. The Xevo TQ-S tandem quadrupole mass spectrometer is equipped with novel StepWave transfer optics and large sampling orifice which dramatically increases both quantitative and qualitative assay sensitivity. Here we demonstrate the increase in full-scan sensitivity obtained with this new StepWave design using the metabolism of the common non-steroidal anti-inflammatory (NSAID) drug ibuprofen.
|
LC system: |
ACQUITY UPLC (binary solvent manager, sample manager, HT column oven) |
|
LC column: |
ACQUITY UPLC BEH C18 1.7 μm, 2.1 x 50 mm |
|
Column temp.: |
40.0 °C |
|
Gradient: |
A: 0.1% Ammonium hydroxide (Aq) B: Acetonitrile, 5 to 95% B in 1.5 min |
|
Flow rate: |
600 μL/min |
|
Injection vol.: |
10 μL |
|
MS system: |
Xevo TQ-S and Xevo TQ operated in electrospray negative mode Full-scan data acquisition: Scan Range 200 to 600 m/z MRM data acquisition 205 => 161 |
|
Voltages: |
Capillary, cone, and collision voltage where optimized for each mass spectrometer as well as cone gas flow |
|
Collision energy: |
7 eV MRM acquisition, 3 eV full-scan acquisition |
|
Source temp.: |
140 °C |
|
Desolvation temp.: |
625 °C |
|
Nebuliser gas flow: |
1200 L/hr |
MassLynx 4.1
The detection of low-concentration drug metabolites in biological fluids such as plasma and urine allows informative data to be obtained about a candidate drug discovery molecule from a very low dose to a rat or mouse. However, this requires an analytical system capable of acquiring high sensitivity full-scan data. While tandem quadrupole instruments are the most sensitive instruments for acquiring quantitative data, via multiple reaction monitoring (MRM) mode, they are usually significantly less sensitive in full-scan mode. This means that the acquisition of the necessary qualitative and quantitative data normally requires two analytical runs on two separate instruments. This reduces productivity.
The Xevo TQ-S is a new tandem quadrupole MS equipped with the latest StepWave ion optics which dramatically increases the efficiency of ion transfer from the ion source to the MS analyzer. The use of a two off-axis, stacked ring electrodes design in the transfer region ensures that only the ions of interest are directed to the analyzer. Complementing this increased ion sampling is the ScanWave technology employed in the collision cell. Here, ions within the collision cell accumulate and are then separated according to their mass-to-charge (m/z) ratio. By synchronizing the release of these ions with the scanning of the second quadrupole mass analyzer significantly improves the overall duty cycle of the instrument and hence product ion spectral sensitivity.
The data in Figure 1 shows the comparative full-scan negative ion sensitivity obtained from a tandem quadrupole instrument using the conventional transfer optics and that obtained with the new StepWave ion optics. In this example, urine was collected from a human volunteer two hours after a 100 mg oral dose of ibuprofen. The urine was then diluted 1:1000 with water and injected onto the chromatography system. In this data set we can see the extracted ion chromatograms for the three major classes of ibuprofen metabolites. These are clearly visible in the new StepWave-enabled mass spectrometer whereas they remain undetected in the conventional instrument.
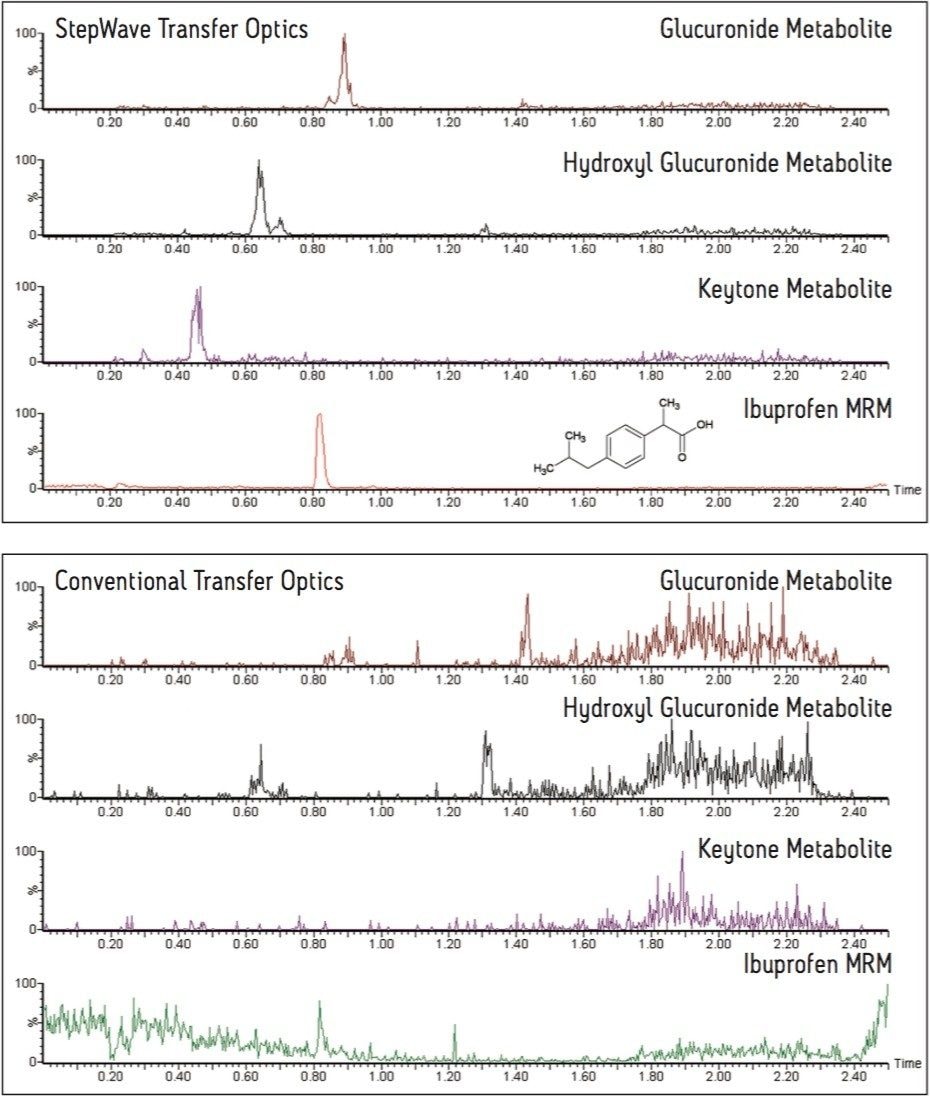
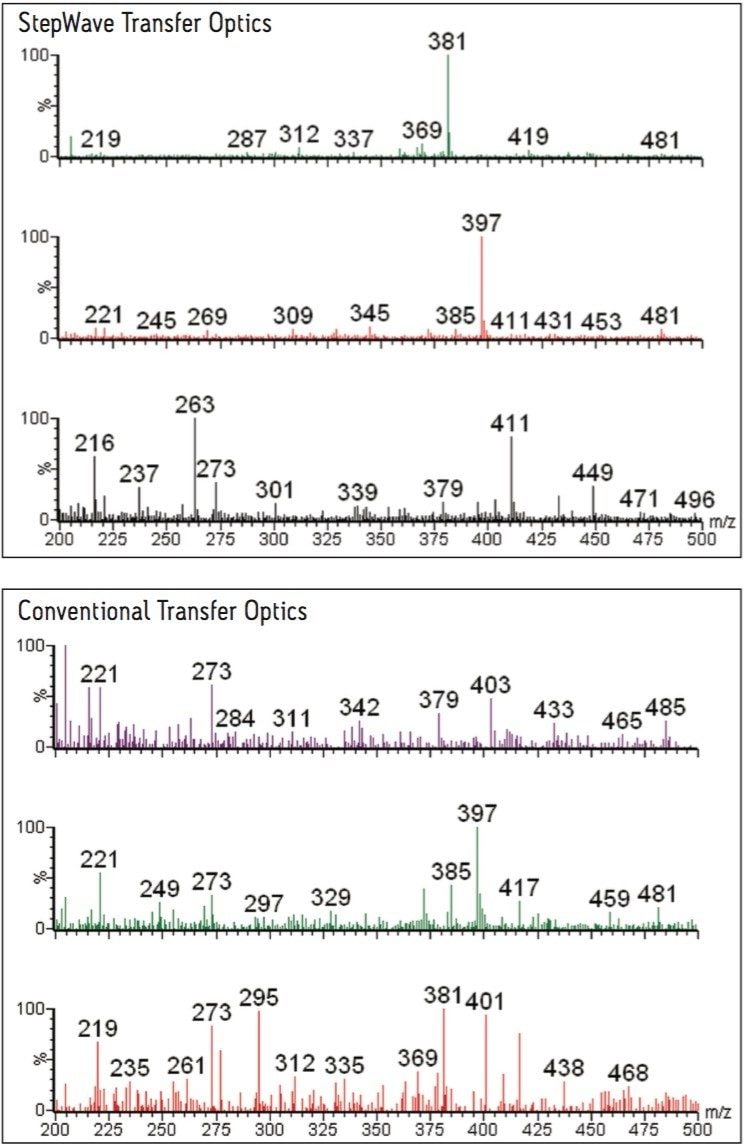
A comparison of the full-scan spectra obtained from the new instrument design and that achieved with an instrument equipped with conventional ion optics is shown in Figure 2. This example demonstrates that the new instrument is capable of acquiring high-quality, full-scan MS spectra for the three types of metabolites whereas this was not possible with the instrument equipped with conventional ion optics. The spectrum with the m/z 411 peak corresponds to the ketone glucuronide, the m/z 397 peak is from the hydroxyl glucuronide, and the m/z 381 peak is from one of the glucuronide metabolites of ibuprofen.
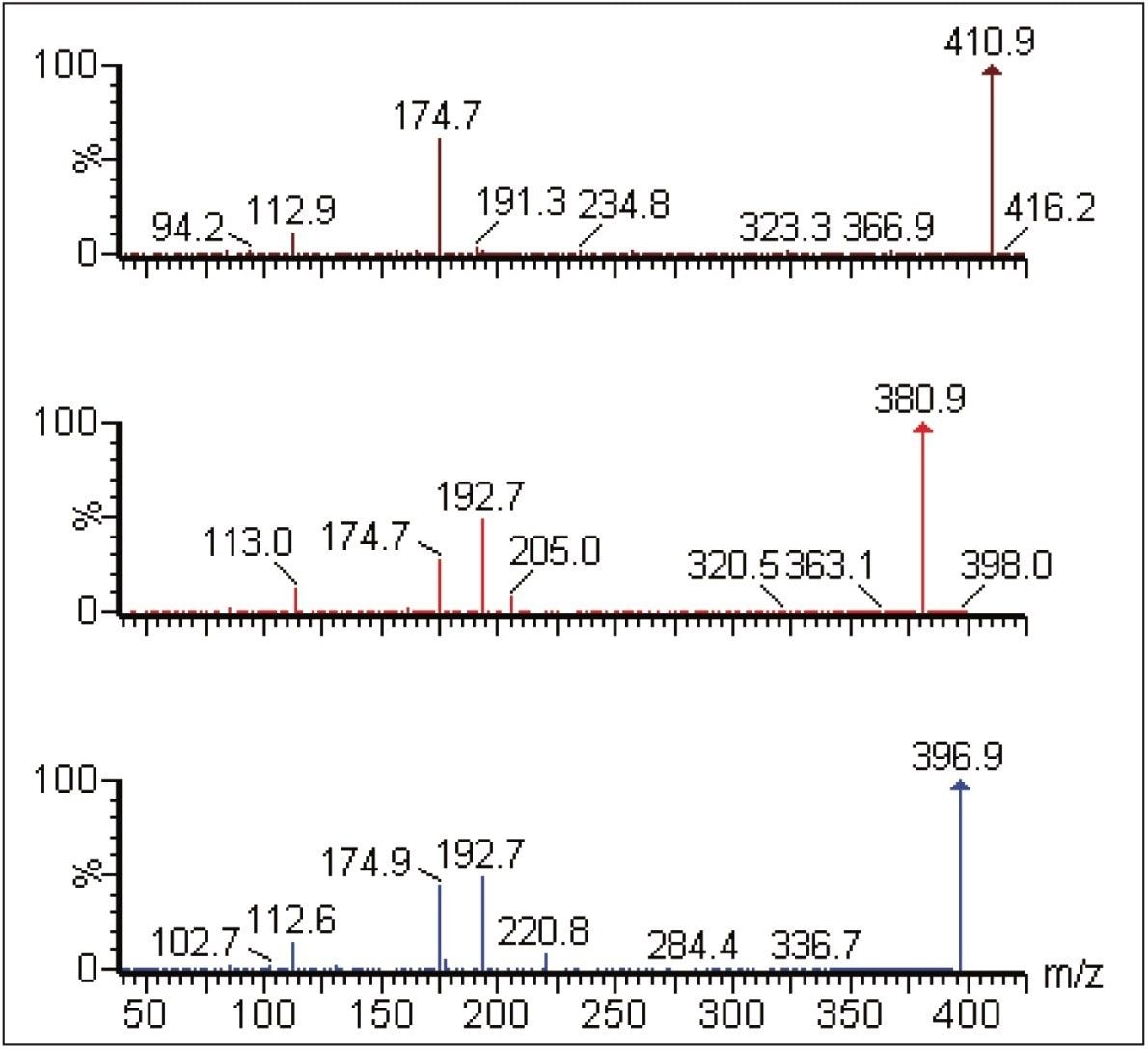
This enhanced sensitivity of the new Xevo TQ-S not only facilitated the detection of the metabolites and collection of the full-scan MS data but also was sufficiently sensitive to allow MS/MS data to be acquired, as shown in Figure 3. The glucuronide metabolite m/z 381 gave rise to three fragment ions, m/z 205, 192, and 113, whereas the hydroxyl glucuronide, m/z 397, gave rise to the product ions, m/z 221, 192, and 113. The keytone glucuronide, m/z 411, gave rise to two major fragment ions, m/z 174 and 113.
The rapid detection and identification of drug metabolites in biological fluids plays a critical role in drug discovery. The ability to detect and characterize these metabolites from low-dose rodent studies can allow studies to be performed with less compound earlier in the discovery process and hence reduce costs. The Xevo TQ-S is a new tandem quadrupole mass spectrometer equipped with the latest ion transfer optics enabling metabolites to be detected at extremely low levels. The Xevo TQ-S is also equipped with RADAR, which allows the simultaneous acquisition of quantitative MRM and qualitative full-scan data in one analytical run. These novel features of the Xevo TQ-S allow:
720003416, May 2010