1. Determine the precision and accuracy of quantification of choline by DIOS-Tof MS and compare to LC-MS/MS
2. Measure the acetylcholine esterase Km by DIOS-Tof MS
3. Find the IC50 for tacrine inhibition of the acetylcholine esterase reaction
4. Compare the DIOS-Tof MS and LC-MS/MS methods for analysis of enzyme-inhibition reactions
The DIOS-Tof MS method is shown to a be rapid, simple approach to relative quantification for enzyme-inhibition IC50 assays
The pharmaceutical industry analyzes enzymeinhibition reactions to determine the efficacy of a wide array of inhibitors. The ideal analytical technique for such work should be both quantitative and capable of a fast sample cycle time. A common example of enzyme-inhibition analysis is found in the case of Alzheimer’s disease1,2 where acetylcholine esterase inhibitors have been shown to lessen the effects of the disease. Inhibitors have been tested using traditional methods including microtiter plate-based fluorescence assays,3 liquid chromatography (LC) mass spectrometry (MS)4,5 and LC with UV absorption or fluorescence detection.4,6 The fastest cycle times areachieved with the plate-based fluorescence assays though this method has limited analyte specificity. LC-MS-based approaches provide highly specific information but tend to have longer cycle times. Laser desorption based methods7-10 have been used extensively for small molecule quantification including the MALDI based methods7,9,11-16 and the matrix-free method of desorption/ionization on silicon (DIOS).8,10,17,18 These approaches offer the potential for rapid cycle time combined with mass selectivity. The DIOS-Tof MS method is used in this work to quantify the rate of production of the choline product of the acetylcholine esterase reaction in the presence of the inhibitor tacrine. The results are compared between the DIOS-based method and the standard LC-MS/MS approach.
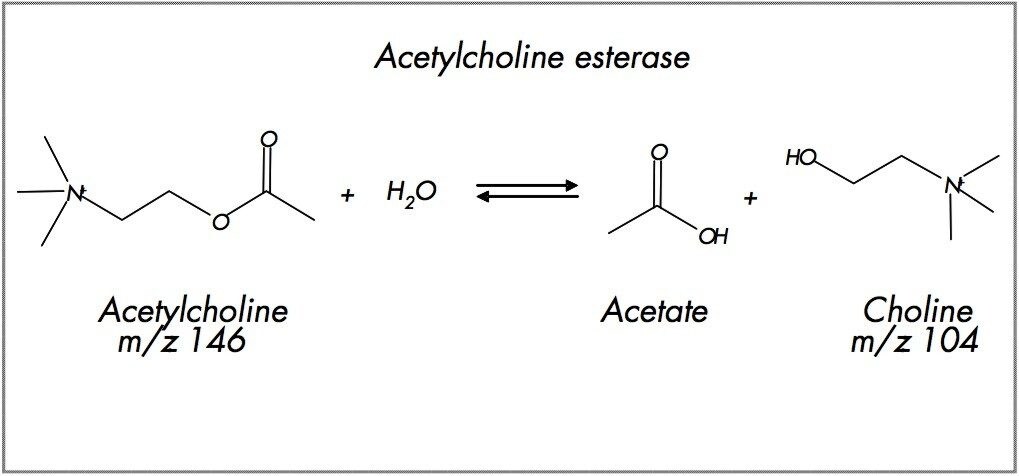
The enzyme reaction was typically run in 10mM ammonium bicarbonate and incubated at 37 °C. The enzyme activity was quenched at specific time points by addition of one volume of acetonitrile containing the choline internal standard (choline-d9: 90 μM). This solution was either directly spotted onto the MassPREP DIOS-target Plate (0.4 μL per well) or diluted 10-fold with water prior to LC-MS/MS (25 μL per injection).
96 well MassPREP DIOS-target Plates were analyzed using a Micromass MALDI-Tof MS instrument operating in reflectron mode.
LC-MS/MS analyses were performed on a Waters Micromass Quattro micro equipped with an electrospray interface. The instrument was operated in MRM mode for detection of choline (104.1 → 59.8) and the internal standard, choline-d9 (113.2 → 68.9).
|
LC system: |
Alliance HT 2795 XC Separations Module |
|
Column: |
Waters Atlantis dC18 3 μm 2.1 x 100 mm |
|
Flow rate: |
600 μL/min. split to 150 μL/min. into MS |
|
Injection volume: |
25 μL |
|
Gradient: |
Isocratic at 70% A and 30% B over 2 min. where A = 0.1% Formic Acid in Water, and B = 0.1% Formic Acid in Acetonitrile |
|
MS system: |
Micromass Quattro micro |
|
Ion mode: |
Positive |
|
Cone voltage: |
30 V |
|
Desolvation temp.: |
250 °C |
|
Source temp.: |
120 °C |
|
Collision energy: |
18 V |
|
Detection mode: |
MRM |
|
Dwell: |
80 ms |
|
Collision gas: |
Argon, 2e-3 Torr |
Two critical parameters in the study of enzyme-inhibition reactions are the Michaelis constant (Km) and the fifty percent inhibition constant (IC50). The Km is the substrate concentration at which the reaction occurs at half of the maximum rate and is an indicator of the affinity that an enzyme has for a given substrate. The IC50 is the concentration of inhibitor that reduces the enzyme reaction’s initial velocity by half and is a measure of an inhibitor’s efficacy.
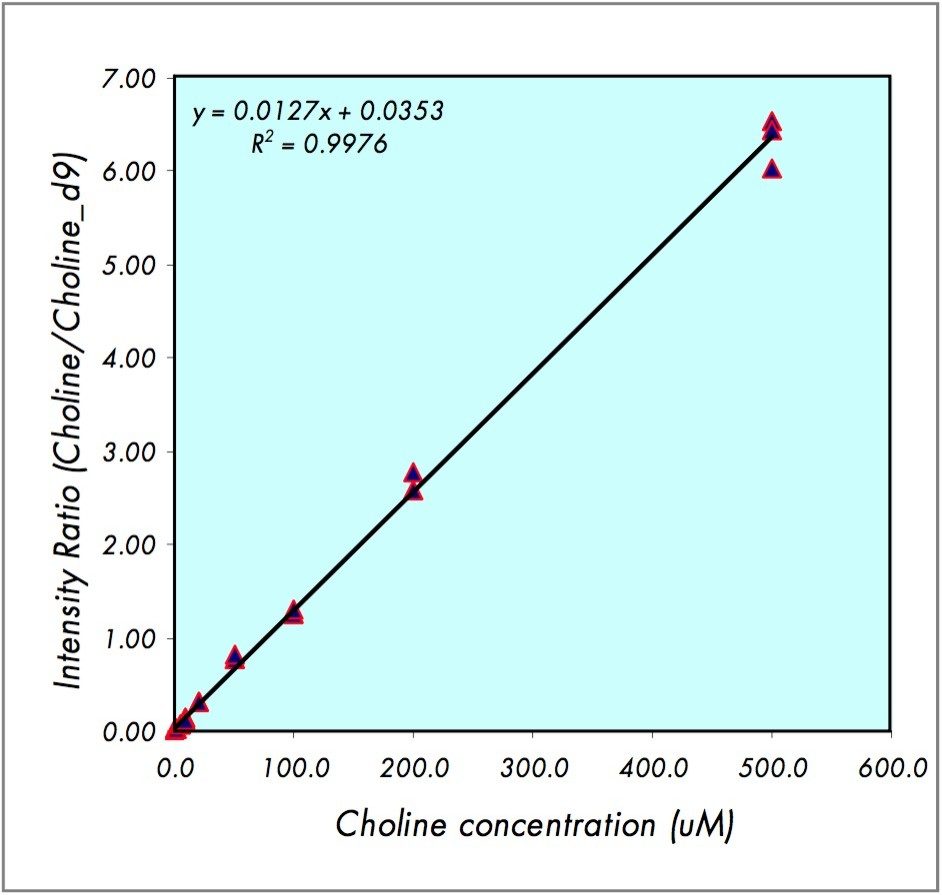
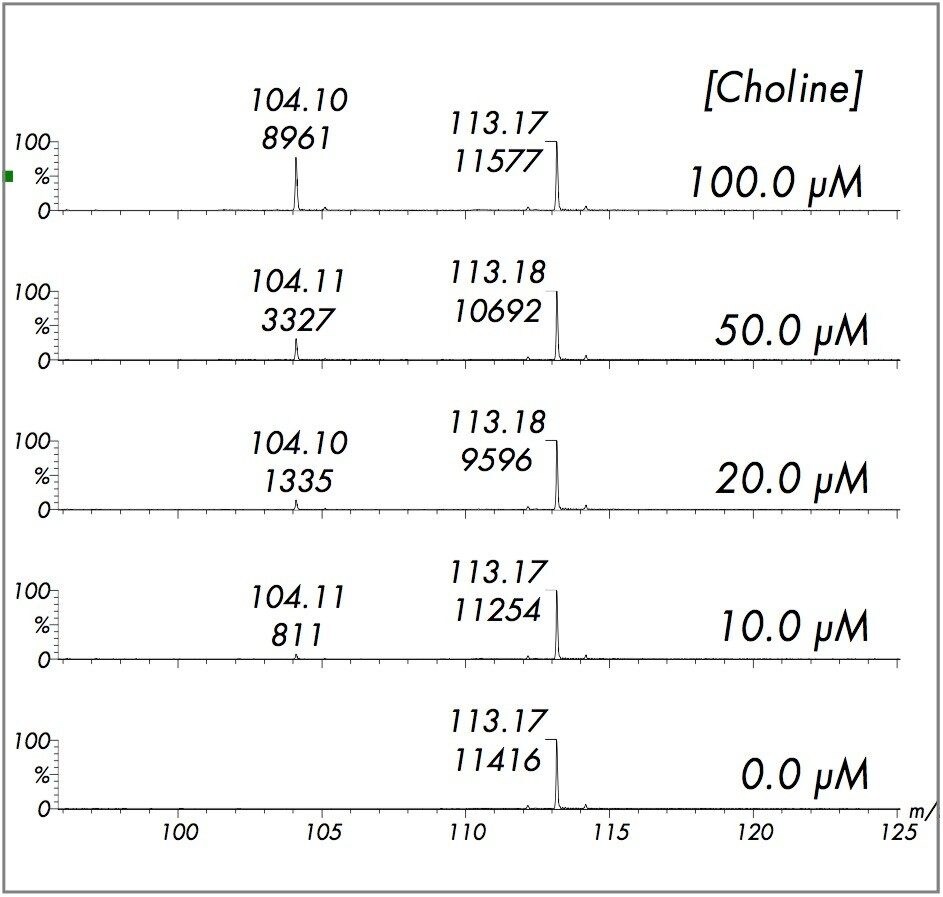
The standard calibration curve for choline is shown in Figure 1. The linear dynamic range extends from 5.0 to 500.0 μM (3.8% RSD, R2 = 0.9976). Examples of the DIOS-Tof MS spectra are shown in Figure 2 for 100.0, 50.0, 20.0, 10.0, and 0.0 μM concentrations of choline. Data were acquired by randomly sampling the target with the nitrogen laser and the final mass spectra were composed of 150 summed shots. The quantification was based on the MS peak intensity ratio of choline to choline-d9.
The standard calibration curve for choline using LC-MS/MS extended from 1.0 μM to 500.0 μM (%RSD 1.6%, R2 = 0.999). The LC-MS/MS method performs better than the DIOS-Tof MS method in terms of linear dynamic range, %RSD and linear regression; however both methods are quantitative, and provide a means of assaying the acetylcholine esterase reaction.
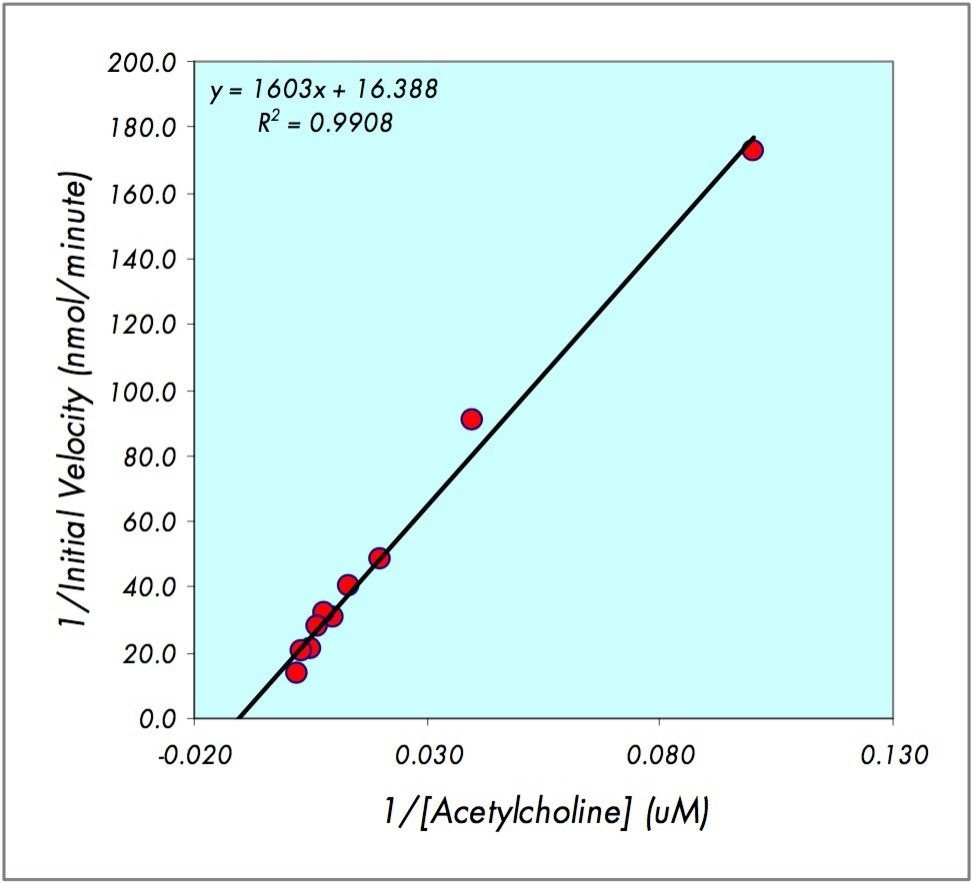
Where V0 is the initial linear velocity of the reaction and [S] is the concentration of substrate, a plot of 1/V0 vs. 1/[S] generates a Lineweaver-Burk plot and a linear regression fit to this data is then used to estimate the Km (Figure 3) at the x-intercept.
With the acetylcholine concentration held at the Michaelis constant the reaction was run in the presence of the inhibitor tacrine at the following concentrations: 0.0, 0.1, 0.2, 0.5, 1.0, 2.0, 5.0, 10.0, 20.0, 50.0, 100.0, 200.0, 500.0, 1000.0, 2000.0, 5000.0, and 10000.0 nM. After quenching the reaction at thirty minutes the concentration of choline was determined. The plot of the concentration of choline vs. the log of the concentration of tacrine is shown in Figure 4 for DIOS-Tof MS and LC-MS/MS. The data clearly show a trend from no inhibition at low concentrations of tacrine to one hundred percent inhibition at high concentrations of tacrine. The midpoint between these two levels represents the fifty percent inhibition level and is used to calculate the IC50.
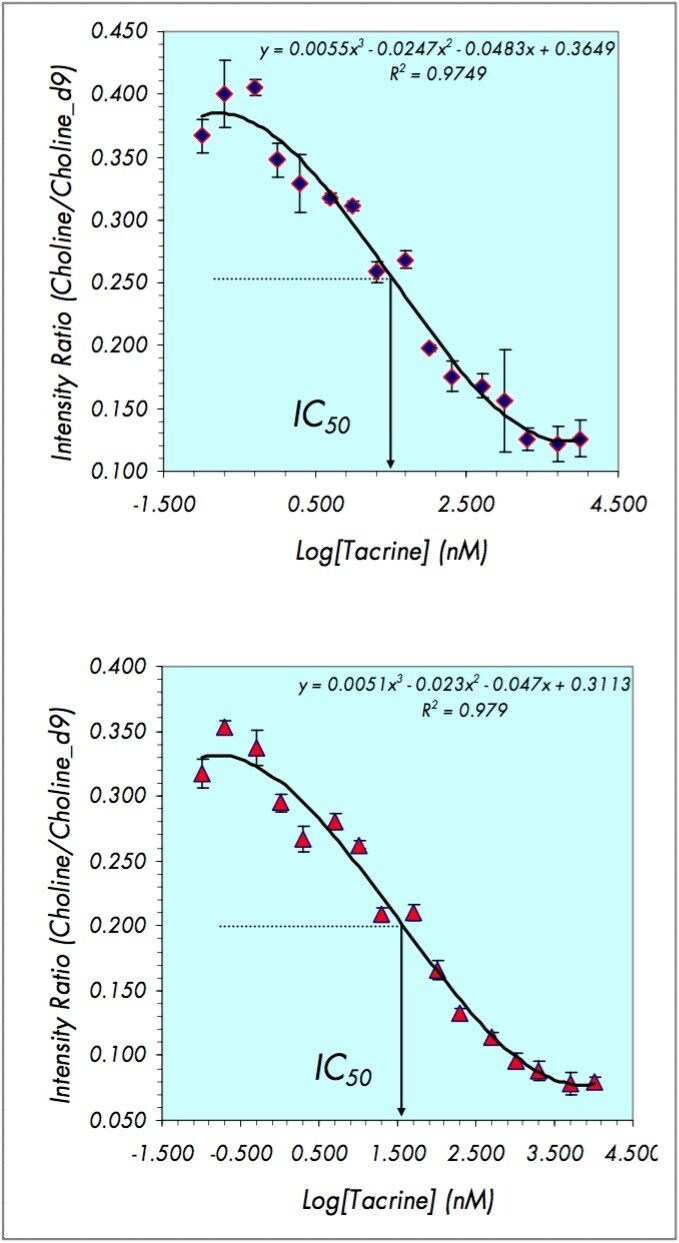
With a third order polynomial fit to this data the concentration of inhibitor required for fifty percent inhibition of the enzyme, (IC50) can be estimated (Figure 4). The DIOS-Tof MS approach yielded a calculated IC50 of 32.0 +/-0.2 nM while the LC-MS/MS method gave an IC50 of 31.8 +/-0.1 nM. The reported IC50 value is 30.0 nM.1 The DIOS-Tof MS and LC-MS/MS methods both result in IC50 values close to the reported value, demonstrating that DIOS is an effective technique for determining IC50 calculations.
Wall, D, Finch J, Cohen C. Comparison of Desorption/Ionization on Silicon (DIOS) Time-of-Flight and Liquid Chromatography Tandem Mass Spectrometry for Assaying Enzyme-Inhibition Reactions. Rapid Commun. Mass Spectrom. 2004; in press.
WA40488, November 2004