Method Development for Forced Degradation of Small Molecule GLP-1 Agonist Orforglipron
Melissa Aiello, Christopher Collins, Thomas H Walter
Waters Corporation, United States
Published on November 26, 2025
Abstract
Orforglipron is a novel non-peptide GLP-1 RA (glucagon-like peptide receptor agonist) that is currently being developed to combat obesity and type II diabetes. This small molecule drug is one of the first GLP-1 RA to be administered orally rather than by injection and is poised to be filed for FDA approval in early 2026 by Eli Lilly. For all pharmaceutical companies it is imperative to deliver safe and effective medication to patients. Within the context of a pharmaceutical analytical laboratory, this is supported by developing impurity methods for the active pharmaceutical ingredient (API) using high performance liquid chromatography (HPLC). This is usually done by stressing the API under different conditions to elucidate degradation pathways and identify impurities in a forced degradation study.
In this work, an LC-MS impurity method was developed for orforglipron based on a forced degradation study. The systematic screening protocol (SSP) was used to develop a method quickly and efficiently. The final method shows excellent peak shape for orforglipron and good separation from the impurities as measured by mass purity.
Benefits
- An impurity method for orforglipron was developed based on a forced degradation study
- The API is spectrally pure with sharp peaks and is well-resolved from all impurities
- Peak shape and resolution meet criteria under USP 621 guidelines
Introduction
Glucagon and glucagon-like peptide 1 (GLP-1) are endocrine hormone analogues that play key roles in glucose metabolism. While both peptide hormones belong to family B of the seven transmembrane (7TM) G-protein coupled receptors,1 they play vastly different roles in glucose metabolism. Glucagon is produced by the liver and α-cells of the pancreas, and during times of fasting promotes gluconeogenesis and glycogenolysis.1 This releases free glucose into the bloodstream during an energy deficit.
GLP-1, on the other hand, is a hormone produced by intestinal epithelial cells that stimulates insulin release and slows gastric emptying in response to a meal. It accomplishes this by interacting with GLP-1 receptors in the pancreatic β-cells,1 causing a decrease in blood sugar levels. Additionally, GLP-1 hormones can affect feelings of hunger in the brain by stimulating GLP-1 receptors in the vagal afferent neuron (VAN) system, a major link between the gastrointestinal tract and central nervous system.2
Today, GLP-1 RAs have garnered international attention for the treatment of type II diabetes and obesity. Currently, there are only two GLP-1 RAs that have been approved specifically for weight management; liraglutide and semaglutide.3 These medicines are administered as an injection and, while effective, can provide a barrier of access to patients through their method of administration. Pharmaceutical companies are racing to develop drugs with easier administration and decreased side effects of traditional injectables. Orforglipron, currently in Phase 3 clinical trials for obesity and diabetes, is a novel small-molecule GLP-1 RA that can be adsorbed by the small intestine, and is one of the first that can be administered orally.3 Additionally, it has a partial agonistic mechanism of action compared to the peptide GLP-1 RA, which can prevent desensitization to the drug over time.3 Orforglipron has the potential to open access to safe and effective diabetes and weight loss treatment to greater populations of patients.
In this application note, an LC-MS impurity method for orforglipron was developed based on a forced degradation study. Forced degradation studies are critical not only for the development of stability-indicating analytical methods for the drug substance and/or drug product, but for elucidating major degradation pathways of the API.4 The analytical method was developed using the SSP. This involves three simple steps: (1) pH scouting, (2) solvent screening, and (3) optimization. The reasoning behind column and solvent selections, combined with the data-driven decision making, will be discussed in shaping the analytical method. Critical parameters of the analytical method, such as analyte peak purity and peak shape, as well as impurity USP resolution and sensitivity, will be discussed.
Experimental
Sample Preparation
A 1 mg/mL solution of orforglipron in 60:40 (v/v) acetonitrile and water was prepared. 0.9 mL of the standard solution was aliquoted into three separate vials (labeled as Acid, Base, and Peroxide). 0.1 mL of the corresponding degradant was added (0.1 mL of 1 N HCl, 0.1 mL of 1 N NaOH, 0.1 mL of 3% H2O2). The acid and base vials were heated to 70 °C for 2 hours, and the peroxide vial was heated to 70 °C for 1 hour. All vials were combined to quench the acid and base, then aliquoted into an LC vial for analysis.
Initial Method Development Conditions
|
LC system: |
ACQUITY™ Premier H-Class with ACQUITY PDA and QDa™ Detectors |
|
Detection: |
MS Full Scan (ESI+) UV @ 254 nm |
|
Column(s): |
ACQUITY Premier BEH™ C18 Column 2.1 x 50 mm, 1.7 µm (p/n: 186002350) ACQUITY Premier BEH C18 Column 2.1 x 100 mm, 1.7 µm (p/n: 186009453) ACQUITY Premier CSH™ Phenyl-Hexyl Column 2.1 x 50 mm, 1.7 µm (p/n: 186009474) ACQUITY Premier CSH C18 Column 2.1 x 50 mm, 1.7 µm (p/n: 186009460) Atlantis™ Premier BEH C18 AX Column 2.1 x 50 mm, 1.7 µm (p/n: 186009366) ACQUITY Premier BEH C8 Column 2.1 x 50 mm, 1.7 µm (p/n: 186010356) |
|
Column temperature: |
30 °C |
|
Sample temperature: |
5 °C |
|
Injection volume: |
1 µL |
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Acetonitrile |
|
Mobile phase C: |
Methanol |
|
Mobile phase D1: |
2% formic acid in water |
|
Mobile phase D6: |
200 mM ammonium hydroxide |
|
Washes and diluent: |
60:40 (v/v) acetonitrile and water |
|
Flow rate: |
0.5 mL/min |
|
Gradient: |
5% of either D1 or D6 was used to maintain a constant modifier concentration. Initial conditions of 5% organic were followed by a linear gradient to 95% organic over 6.86 minutes. The composition was held at 95% organic for 1.14 minutes then returned to the initial conditions and held for 2.28 minutes to re-equilibrate. The total run time was 10.30 minutes. |
Data Management
|
Chromatography software: |
Empower™ Chromatography Data System (CDS) |
Results and Discussion
Analytical method development can be a daunting task for chemists. With so many factors to consider, such as the system configuration and system dispersion, solvents and solvent modifiers, columns, and analyte characteristics, it is difficult to balance robustness with efficiency. The SSP was developed to systematically screen key factors within a single run.5 The results of each screening run dictate conditions for the next step.
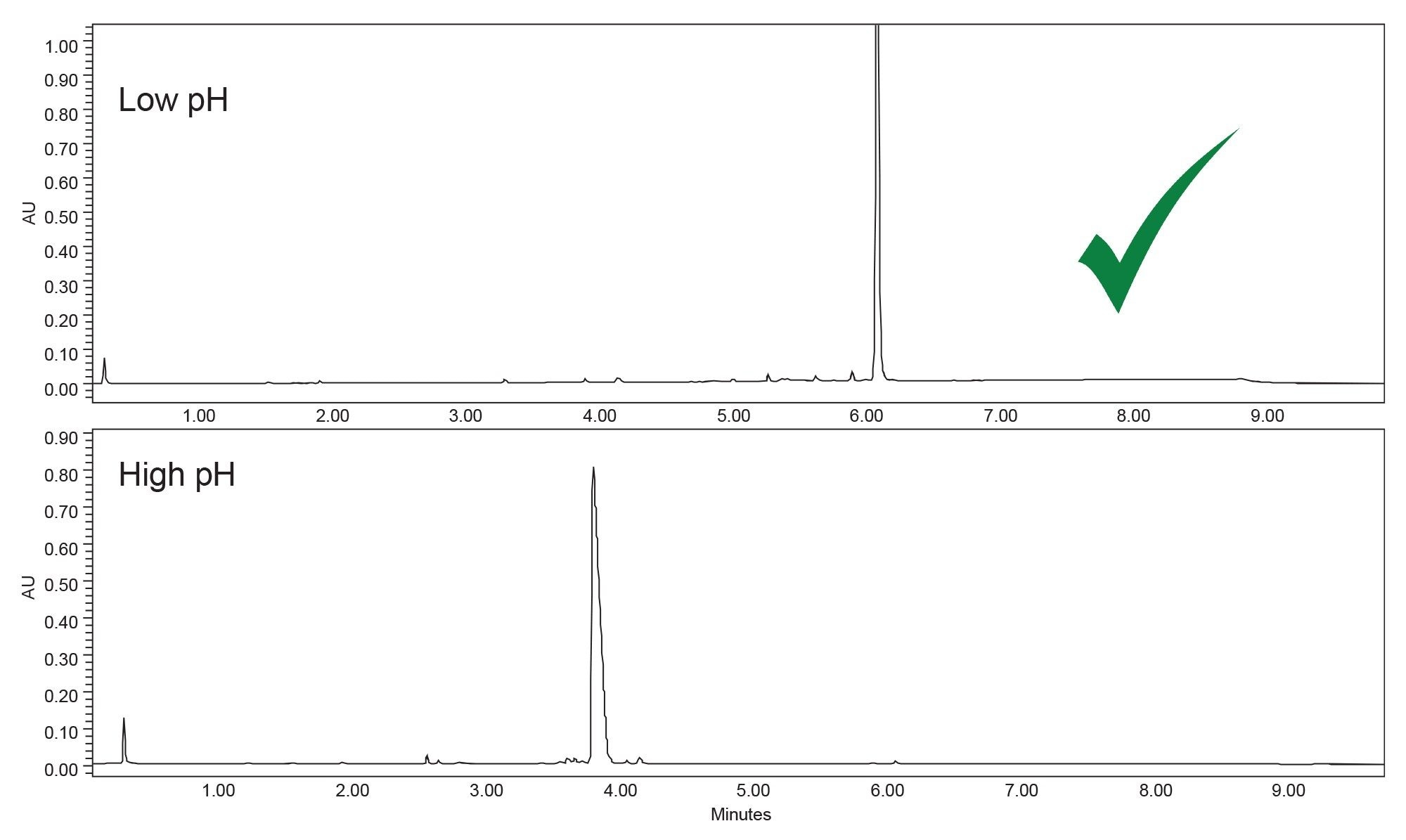
The first step in the systematic screening protocol is to assess retention at high and low pH. This can be accomplished on the instrument by selecting the desired pH modifier at a constant concentration. pH scouting is typically performed using a C18 column with a wide pH stability range, such as the ACQUITY Premier BEH C18 Column. It is crucial to use a column with a wide pH stability range to prevent the column from degrading during testing. With a hybrid organic/inorganic particle containing bridged ethylene groups, the columns are stable up to pH 12. In addition, the trifunctional bonding of the C18 groups imparts exceptional low and high pH stability.
Using the BEH C18 Column, it can be seen in Figure 1 that orforglipron and the impurities are more retained at low pH, with a retention time of approximately 6.2 minutes for orforglipron compared to 3.6 minutes using the basic modifier. Because the analytes of interest are retained better at low pH, 0.1% formic acid in water was used in subsequent method development.
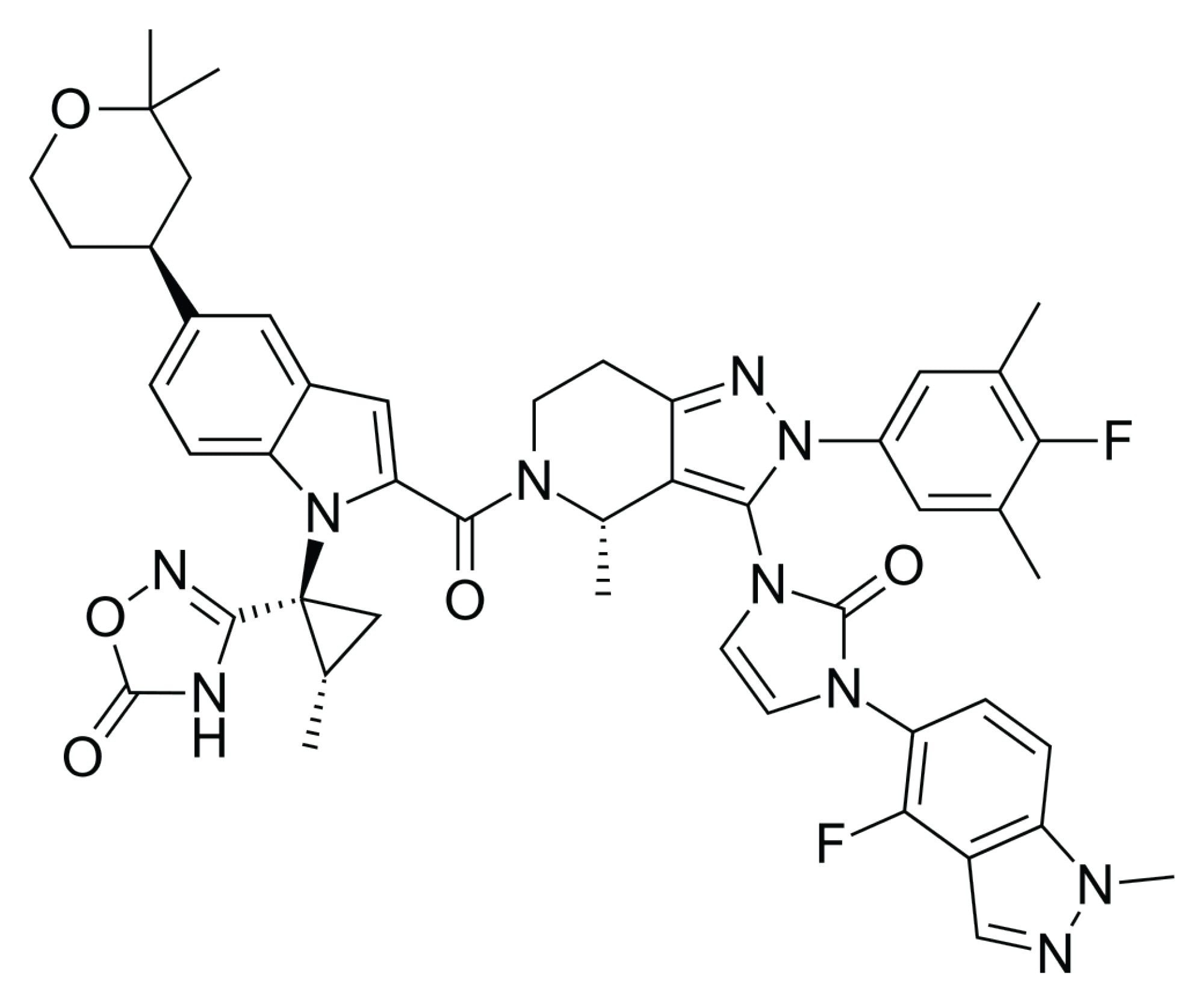
Now that pH scouting is complete, the next step is to assess selectivity. By carefully choosing and screening 4–6 columns using both acetonitrile and methanol as mobile phase solvents, important information regarding analyte selectivity can be determined. Based on the structure of orforglipron, as shown in Figure 2, and in addition to the previously tested BEH C18 Column, an ACQUITY Premier CSH Phenyl-Hexyl Column was chosen. Orforglipron has a lot of aromatic functionality which could enable pi-pi interactions with the Phenyl-Hexyl stationary phase, especially when using methanol as the organic solvent.
Another consideration when choosing columns for screening is the acid/base chemistry of the analyte. Asymmetrical peak shape and low loading capacity for basic analytes is a well-documented issue for many reversed-phase columns when using mobile phases containing formic acid.6 Orforglipron has several secondary and tertiary amines and hence is expected to be slightly basic and could be prone to these chromatographic issues. For this reason, the CSH Phenyl-Hexyl and CSH C18 Columns were chosen. The CSH (charged-surface hybrid) stationary phases contain basic groups that impart a slight positive charge, which can help improve peak shape for basic analytes when using mobile phases containing formic acid. Similarly, the Atlantis Premier BEH C18 AX Column was also selected due to the anion-exchange functionality in addition to the traditional reversed-phase mechanism of the C18.7
Lastly, the ACQUITY Premier BEH C8 Column was chosen as the fifth column. Orforglipron is moderately non-polar and may be retained too strongly on a traditional C18 column. A C8 column offers lower hydrophobic retention, preventing the analyte from excessive interaction with the stationary phase.
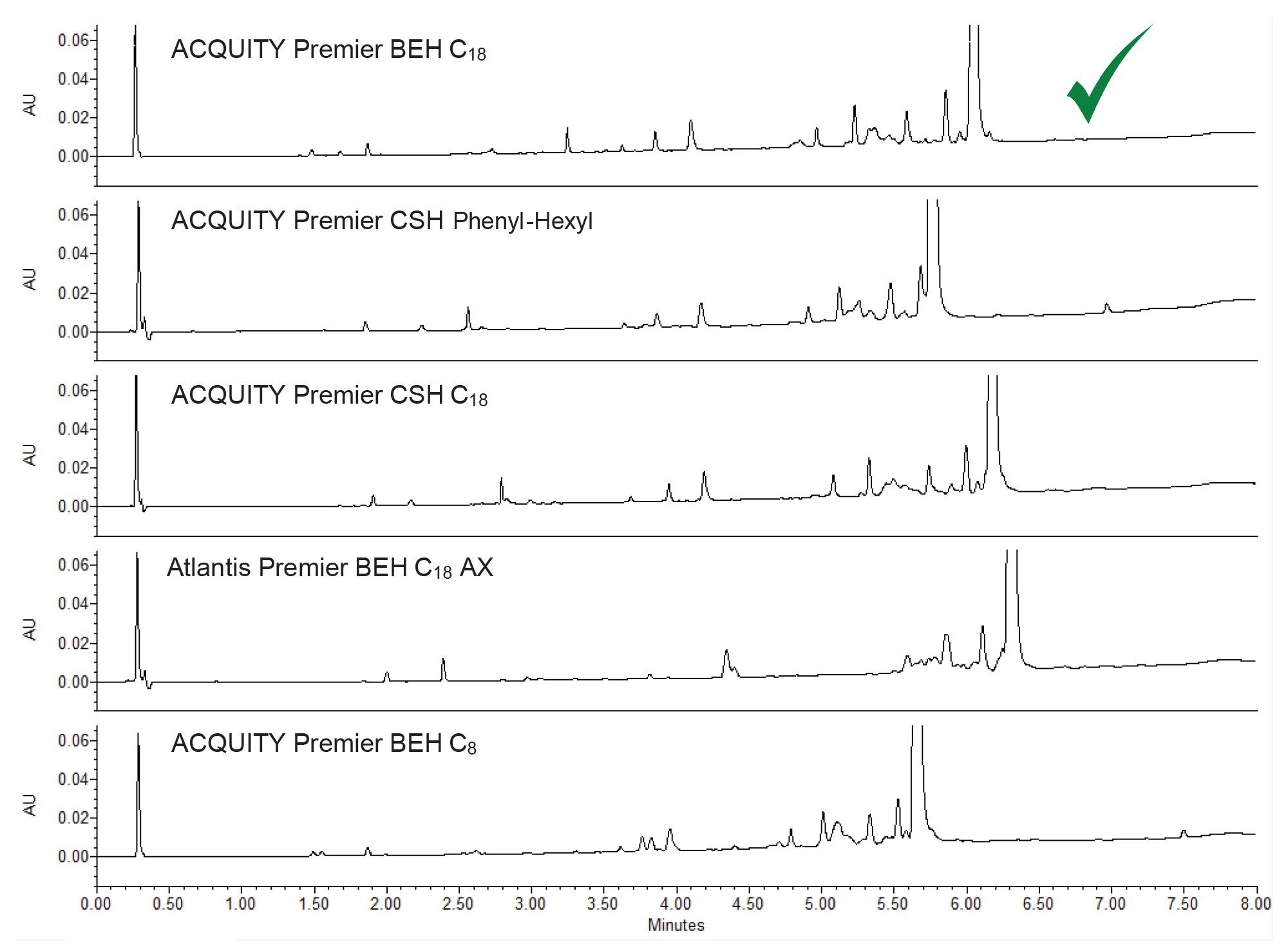
Figures 3 and 4 show the results of column screening using acetonitrile and methanol, respectively, as the strong mobile phase solvent. The goal was to obtain enough selectivity to sufficiently separate orforglipron from close-eluting impurities. When using acetonitrile, the BEH C18 Column and the CSH C18 Column provide the best separations and overall peak shapes. The separations obtained using the CSH Phenyl Hexyl, BEH C18 AX, and BEH C8 Columns had closely eluting impurities peaks slightly before and after orforglipron. The BEH C18 Column sufficiently resolves these impurities and also separates the earlier-eluting impurities from each other.
When using methanol as the strong solvent, while the BEH C18 Column resolved the early eluters from each other, two small impurities eluting before and after the API were not well-resolved. This is also true for the other four columns. Therefore, the ACQUITY Premier BEH C18 Column with acidic modifier and acetonitrile were used going forward.
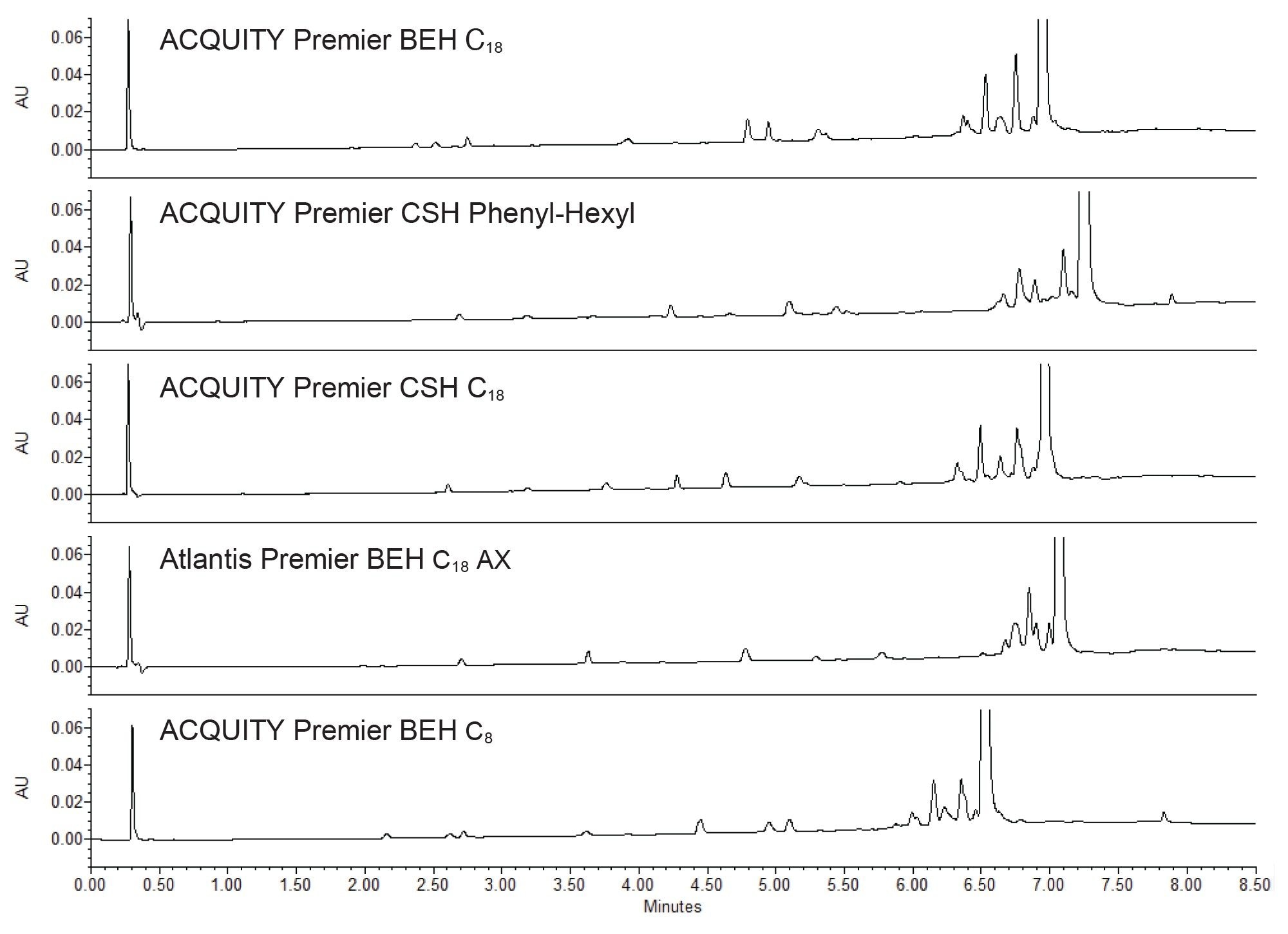
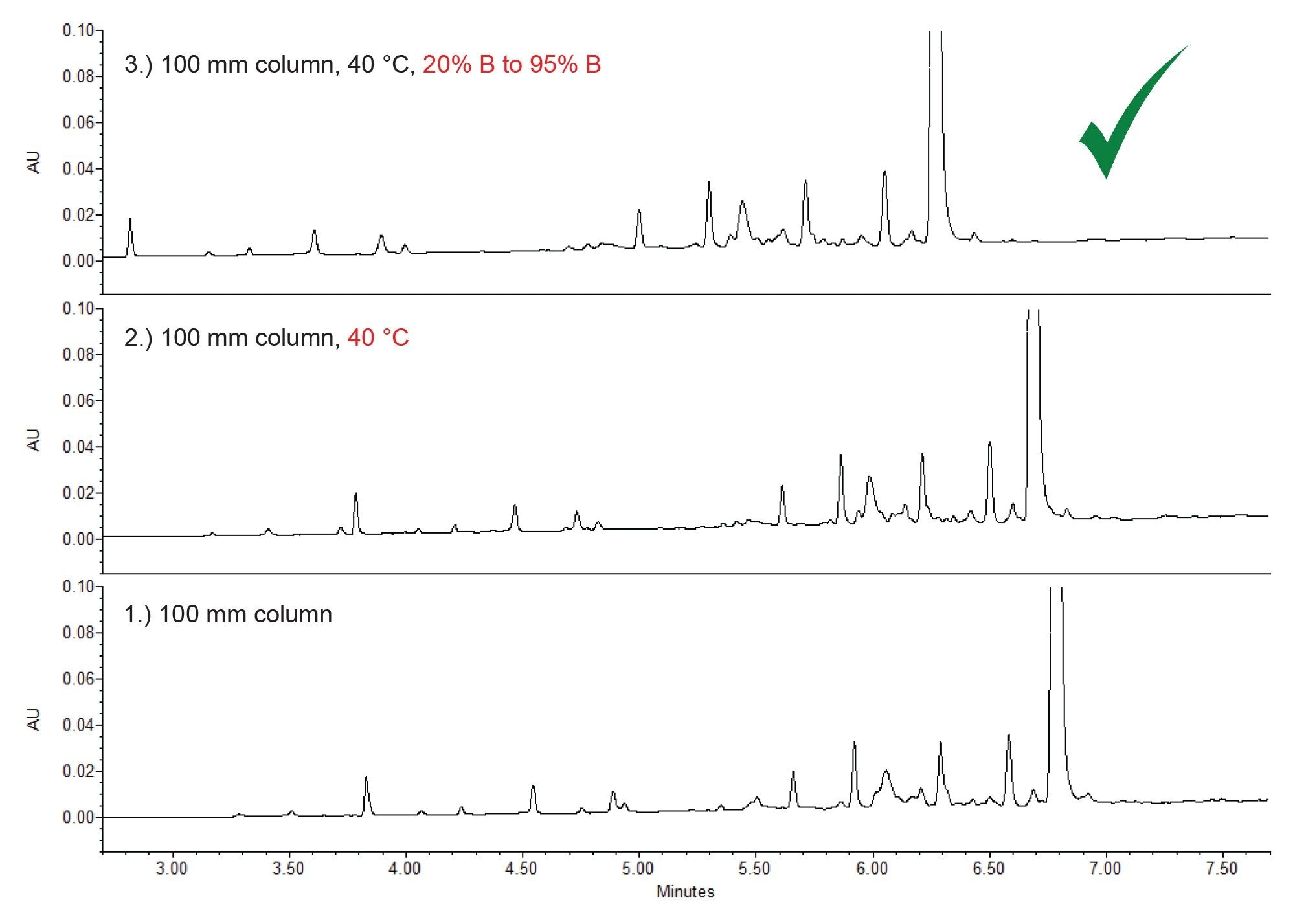
Some parameters that can be considered for optimizing an analytical method are changing the column length, the gradient slope, and the column temperature (Figure 5). To better resolve the impurities closest to orforglipron, a 100 mm length column was employed first. This doubles the column efficiency, improving separation quality. This modification, combined with an increase in column temperature, improved resolution and slightly sharpened the peak shape.
Another tactic is to decrease the gradient slope to make the transition from aqueous to organic more gradual. By starting at 20% B (acetonitrile) instead of 5% in the original screening method, the gradient is shallowed, increasing column-analyte interactions. As shown in the upper chromatogram in Figure 5, this gave a better separation of the impurities and orforglipron, and the closest eluting peaks to the API were better resolved.
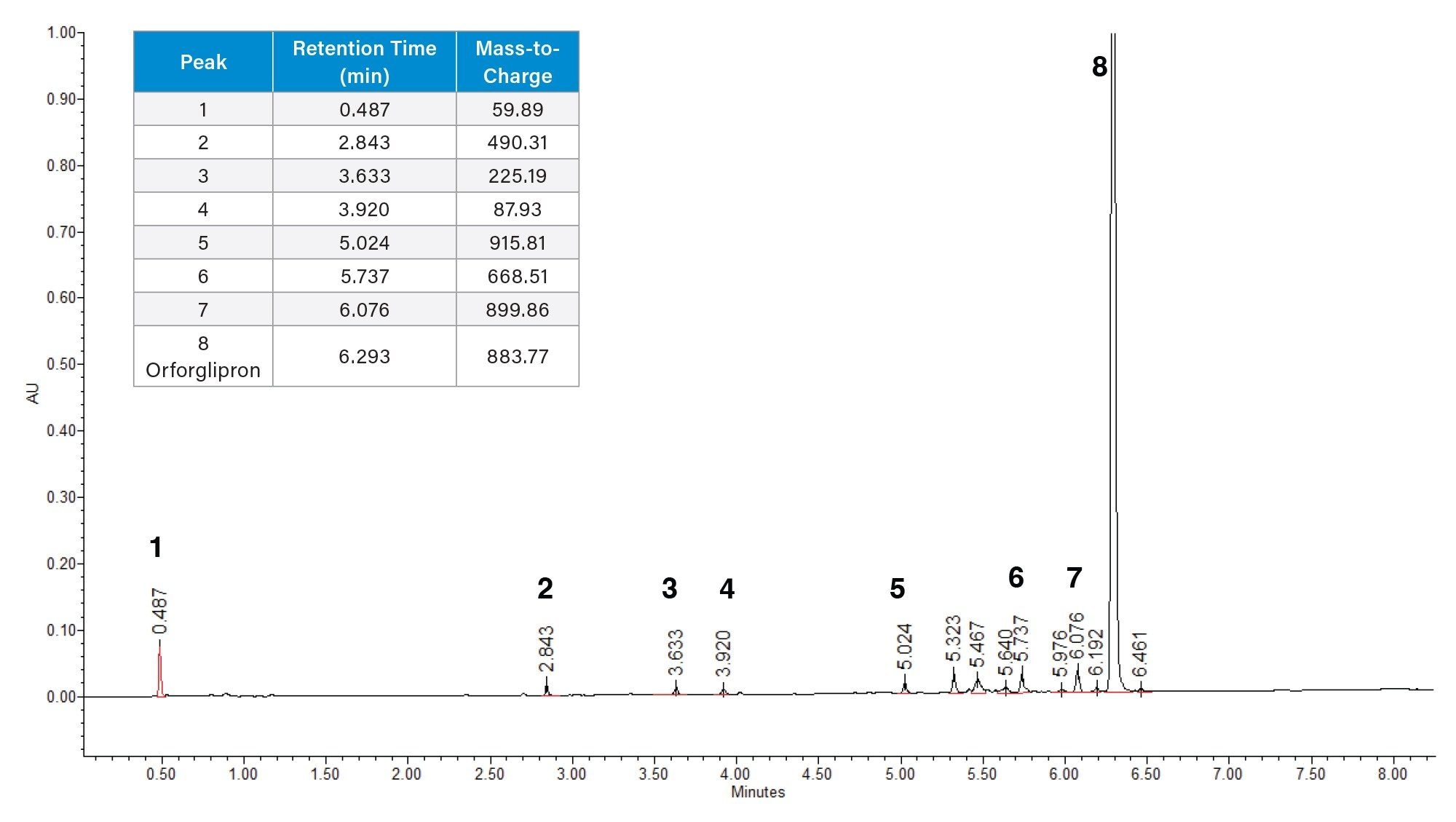
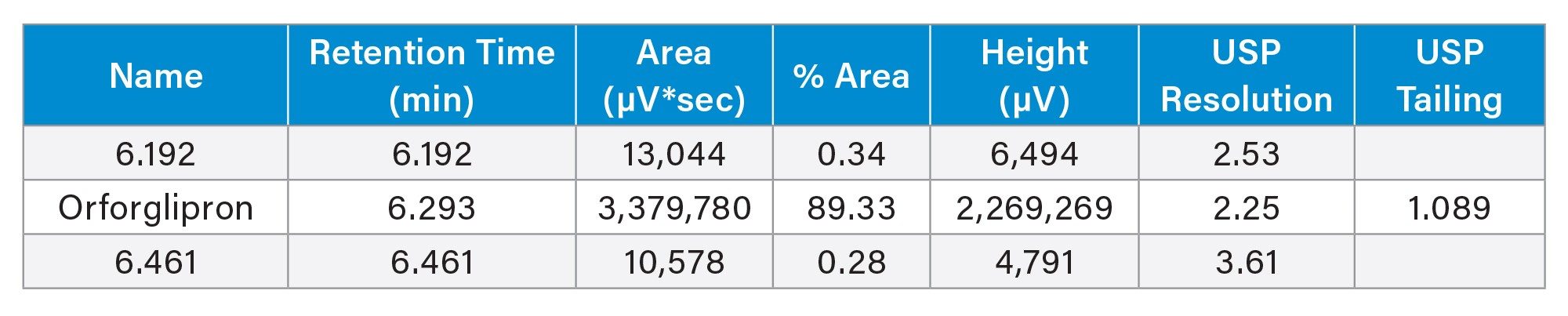
From quality control to developmental laboratories, it is crucial that chromatography for analytical methods follows harmonized standards for retention, peak shape, and resolution. Such guidance is typically provided by a standardization body such as the USP in the United States. According to USP 621 guidelines, analyte peak resolution should be no less than 2.0, and peak tailing (symmetry) should be between 0.8 and 1.8. As shown in Table 1, orforglipron resolution meets this criterion with a resolution of 2.5 and 3.6 from the two closest eluting impurities at 6.192 minutes and 6.461 minutes, respectively. Orforglipron has good peak shape with a tailing factor of 1.1. The analytical impurity method is therefore suitable under USP guidelines.
Another essential parameter to look at in the development of an impurity method is the API peak purity. This can be done spectrally using the UV PDA scan function or by the mass spectrometer. In this case, orforglipron peak purity was determined using the QDa Mass Detector. In positive scan mode, mass spectral data was collected at the leading edge, apex, and trailing edge of the orforglipron peak (Figure 7).
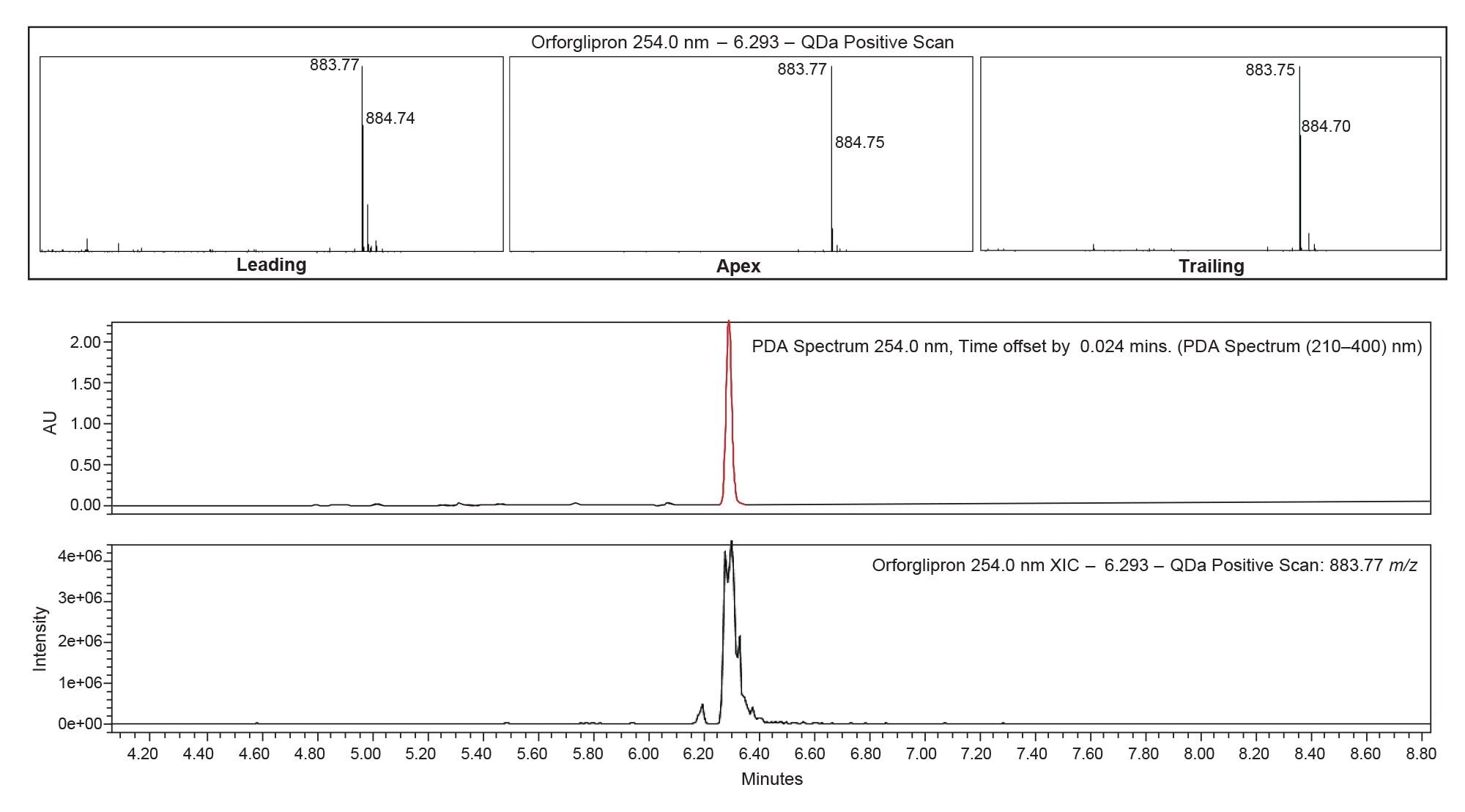
Looking at the upper window of Figure 7, it is evident that there are no other ion species other than the API target ion of approximately 884 m/z for the leading, apex and trailing portions of the peak, other than typical isotopic signatures. This indicates that the orforglipron peak is spectrally pure, and that there are no co-eluting species.
Conclusion
GLP-1 receptor agonists have revolutionized the treatment and management of metabolic diseases such as Type II diabetes and obesity. With the race by pharmaceutical companies to develop and bring them to market, it is crucial to optimize how they are tested in the analytical laboratory. This helps analysts to develop quality chromatographic methods and have confidence in the accuracy and reproducibility of their data.
In this application note, an impurity method was developed for the small-molecule GLP-1 RA orforglipron based on a forced degradation study. The method was developed quickly and efficiently using the SSP in three straightforward steps. The resulting chromatography demonstrated excellent analyte retention, resolution of the API from impurities, and superlative peak shape. The API peak was determined to be spectrally pure and met the peak shape and resolution requirements recommended by USP 621 guidelines.
References
- Runge, S., Wulff, B. S., Madsen, K., Bräuner-Osborne, H., & Knudsen, L. B. (2003). Different domains of the glucagon and glucagon-like peptide-1 receptors provide the critical determinants of ligand selectivity. British Journal of Pharmacology, 138(5), 787–794.
- Ronveaux, C. C., Tomé, D., & Raybould, H. E. (2015). Glucagon-Like Peptide 1 Interacts with Ghrelin and Leptin to Regulate Glucose Metabolism and Food Intake through Vagal Afferent Neuron Signaling. The Journal of Nutrition, 145(4), 672–680.
- Wharton, S., Blevins, T., Connery, L., Rosenstock, J., Raha, S., Liu, R., Ma, X., Mather, K. J., Haupt, A., Robins, D., Pratt, E., Kazda, C., & Konig, M. (2023). Daily Oral GLP-1 Receptor Agonist Orforglipron for Adults with Obesity. The New England Journal of Medicine, 389(10).
- Zelesky, T., Baertschi, S. W., Foti, C., Allain, L. R., Hostyn, S., Juçara Ribeiro Franca, Li, Y., Marden, S., Mohan, S., Ultramari, M. A., Huang, Z., Adams, N., Campbell, J. M., Jansen, P. J., Dorina Kotoni, & Laue, C. (2023). Pharmaceutical Forced Degradation (Stress Testing) Endpoints: A Scientific Rationale and Industry Perspective. Journal of Pharmaceutical Sciences, 112(12).
- Hong, P, McConville, P. A Complete Solution to Perform a Systematic Screening Protocol for LC Method Development. Waters White Paper. 720005268. 2018.
- Iraneta P, Wyndham K, McCabe D, Water T H. A Review of Waters Hybrid Particle Technology Part 3. Charged Surface Hybrid (CSH) Technology and Its Use in Liquid Chromatography. Waters White Paper. 720003929. 2024.
- Walter TH, Alden BA, Boissel C, Field J, Lawrence N, Osterman D, Patel A, A New Mixed-Mode Reversed-Phase/Anion-Exchange Stationary Phase Based on Hybrid Particles, Waters application note. 720006742. 2020.
720009138, November 2025