Targeted and Untargeted Screening of ‘e-liquids’ Using the High Resolution ACQUITY™ RDa™ Mass Detector
Introduction
Electronic nicotine delivery systems (ENDS), also known as ‘vapes’ or e-cigarettes’ are marketed as a healthier alternative to conventional tobacco smoking to reduce health risks and to aid with smoking cessation.1
These products use an ‘e-liquid’ which contains varying levels of nicotine derived from tobacco commonly ranging between 0–20 mg/mL dissolved in a combination of propylene glycol and vegetable glycerin. Flavorings are also added to increase the palatability of the e-liquid. The e-liquid is then heated to create an aerosol which is inhaled.
Although generally regarded as safer than cigarettes due to lower levels of smoke related toxins, e-cigarettes are not completely risk-free. They can still contain nicotine, which is addictive, and other potentially harmful compounds.
Nicotine’s toxicity has been extensively studied and is well understood, with established analytical methods in place to identify and quantify this addictive alkaloid.2,3,4 There is however, a growing interest in understanding the potential negative health impacts from the chemicals used to formulate the bulk e-liquid. Concerns around quality control in the manufacture of these products have been raised since e-liquids are not manufactured to the quality control standards of medications or drug delivery devices.5
There is particular focus on carbonyl containing compounds, which have the potential to be themselves harmful on inhalation. When heated, carbonyl containing compounds can produce carboxyl radicals, a form of reactive oxygen species (ROS).6,7 Therefore, there is a need for routine screening and characterization of the chemicals present in e-liquids.
With the ACQUITY RDa Mass Detector and the UNIFI™ screening platform utilizing in-built libraries, routine screening and compound characterization is achievable without the need for HRMS (High Resolution Mass Spectrometry) expertise.
Within this application note, focusing on the bulk matrix of the product, a commercially available nicotine-free e-liquid was screened against the Waters™ extractables and leachables (E and L) library. The samples were analyzed by liquid chromatography coupled to the ACQUITY RDa Time-of-flight Mass Spectrometer. The acquisition was carried out in both positive and negative electrospray ionization mode. The simultaneous acquisition of high and low energy, Full Scan with Fragmentation, enabled the use of acquired fragment information which increased compound identification confidence.
Major components were identified by mass accuracy measurement <5 ppm. The identification was confirmed by precursor accurate mass and fragments generated in the high energy scan. For unidentified compounds, the Elucidation Toolset feature in UNIFI was used. Hedione is putatively present in the samples. Hedione is a common fragrance/flavoring agent with a well understood safety profile for these applications, but no safety data exists for inhalation.
Benefits
- Routine access to sub 5 ppm mass accuracy HRMS (High Resolution Mass Spectrometry) screening for e-liquid analysis
- Use of in-source fragmentation source generate pseudo MSE spectra
- Use of simultaneous high and low energy acquisition providing component fragment data for additional characterization confidence
- Compliant ready UNIFI HRMS software as part of the waters_connect™ Software Solution
- In built screening workflows for automatic acquiring, processing, and reporting of results
- Use of screening libraries for automatic data interpretation and visualization
- Access to online libraries to investigate unknowns
Experimental
Sample Description
A commercially available strawberry flavoured e-liquid (0 mg/mL nicotine) was purchased from an online retailer. An aliquot was weighed into sample vial and diluted to a concentration 0.5% w/v with mobile phase (98:2, A:B). The sample was analyzed using an ACQUITY RDa Detector in positive and negative mode electrospray and screened against the Waters E and L screening library containing intact and fragment mass information.
LC Conditions
|
LC system: |
ACQUITY UPLC™ I-Class Premier |
|
Vials: |
TruView™ Max Recovery Vials, (p/n: 186005668CV) |
|
Column: |
ACQUITY Premier BEH™ C18 100 x 2.1 m, 1.7 µm (p/n: 186009453) |
|
Column temperature: |
70 °C |
|
Sample tempeature: |
10 °C |
|
Injection volume: |
5 µL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
2 mM ammonium formate/0.1% formic acid |
|
Mobile phase B: |
Methanol/0.1% formic acid |
|
Gradient: |
2 to 99% B/8.5 minutes |
|
Needle wash: |
95:5 MeOH : Acetone |
MS Conditions
|
MS system: |
ACQUITY RDa Mass Detector |
|
Ionization mode: |
ESI Positive |
|
Acquisition range: |
50–2000 m/z |
|
Capillary voltage: |
1.00 kV |
|
Cone voltage: |
20 V |
|
Full scan with fragmentation: |
60–150 V |
|
Desolvation temperature: |
500 °C |
|
Ionization mode: |
ESI Negative |
|
Acquisition range : |
50–2000 m/z |
|
Capillary voltage: |
0.8 kV |
|
Cone voltage: |
40 V |
|
Full scan with fragmentation: |
60–150 V |
|
Desolvation temperature: |
500 °C |
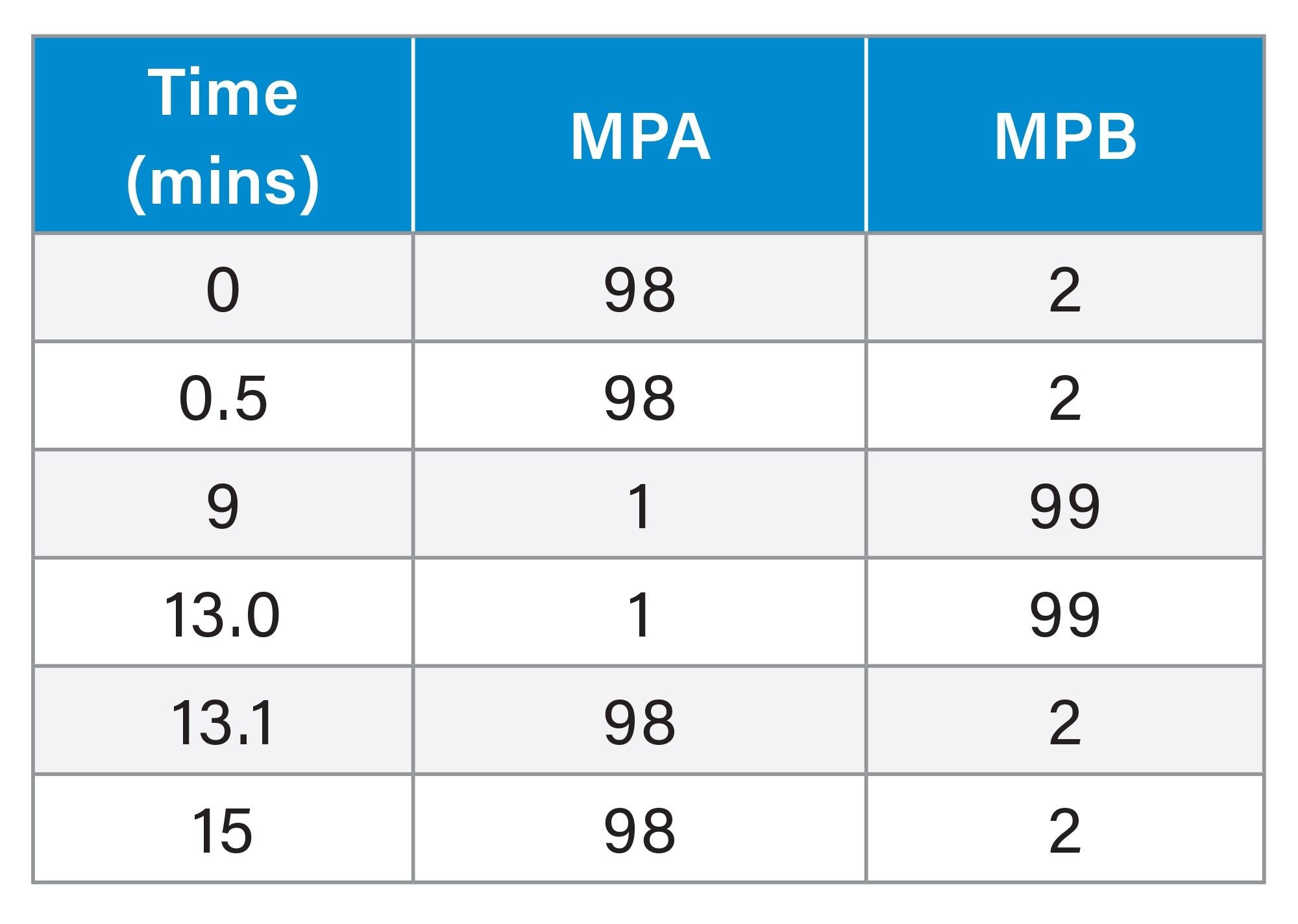
Gradient Table
Data Management
|
Chromatography software: |
UNIFI version 3.6.0.21 waters_connect version 4.1.0.17 |
Results and Discussion
Screening Workflow
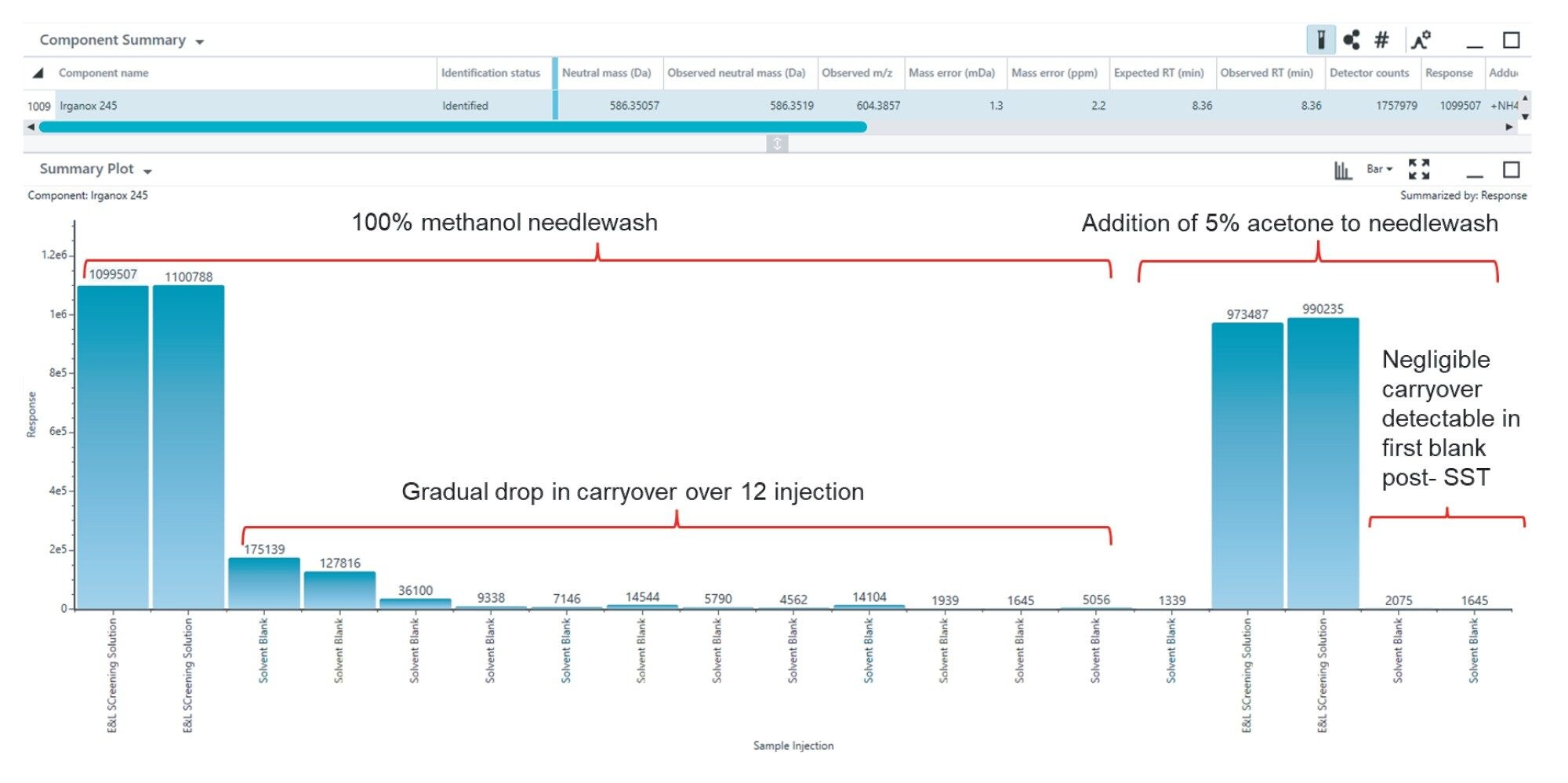
As E and L samples cover a wide range within a single sample, carryover can be an issue for highly retained compounds. Two main parameters were optimized during the method development. First, the column temperature was increased. The BEH C18 Column can operate up to 80 °C under acidic conditions . As this column chemistry can operate at high temperatures without loss of performance, in this application, the column temperature was increased from 40 °C to 70 °C.8 This improved the separation and the peak shapes (data not shown). The development of this method began with optimization of the chromatographic conditions based on the system suitability solution. Secondly, in order to mitigate the sample-to-sample carry over different needle wash solvents were tested as part of the method development process. Irganox 245, used as an antioxidant, demonstrated significant carryover evident in mobile phase blanks run after these samples. Modifying the existing 100% methanol needle wash with the addition of 5% acetone significantly reduced the carry over to a negligible level (Figure 1). This mix of methanol/acetone (95/5, v/v) was chosen as the needlewash for this analysis.
Using the Full Scan with Fragmentation function allows cone voltage ramping to simultaneously acquire high and low energy spectra. UNIFI processes this information as data independent analysis, displaying the spectra in two channels: low collision energy and high collision energy. The accurate mass in the low collision energy is used in the first step of screening by accurate mass. When a potential candidate is assigned, the high energy data function, containing fragment ion information, is then used to confirm the assignment providing further confidence for compound identification. Some library entries did not contain fragment information therefore, corroboration on fragment information was obtained from external sources.9,10 The average mass accuracy over the five injections of any components identified was measured.
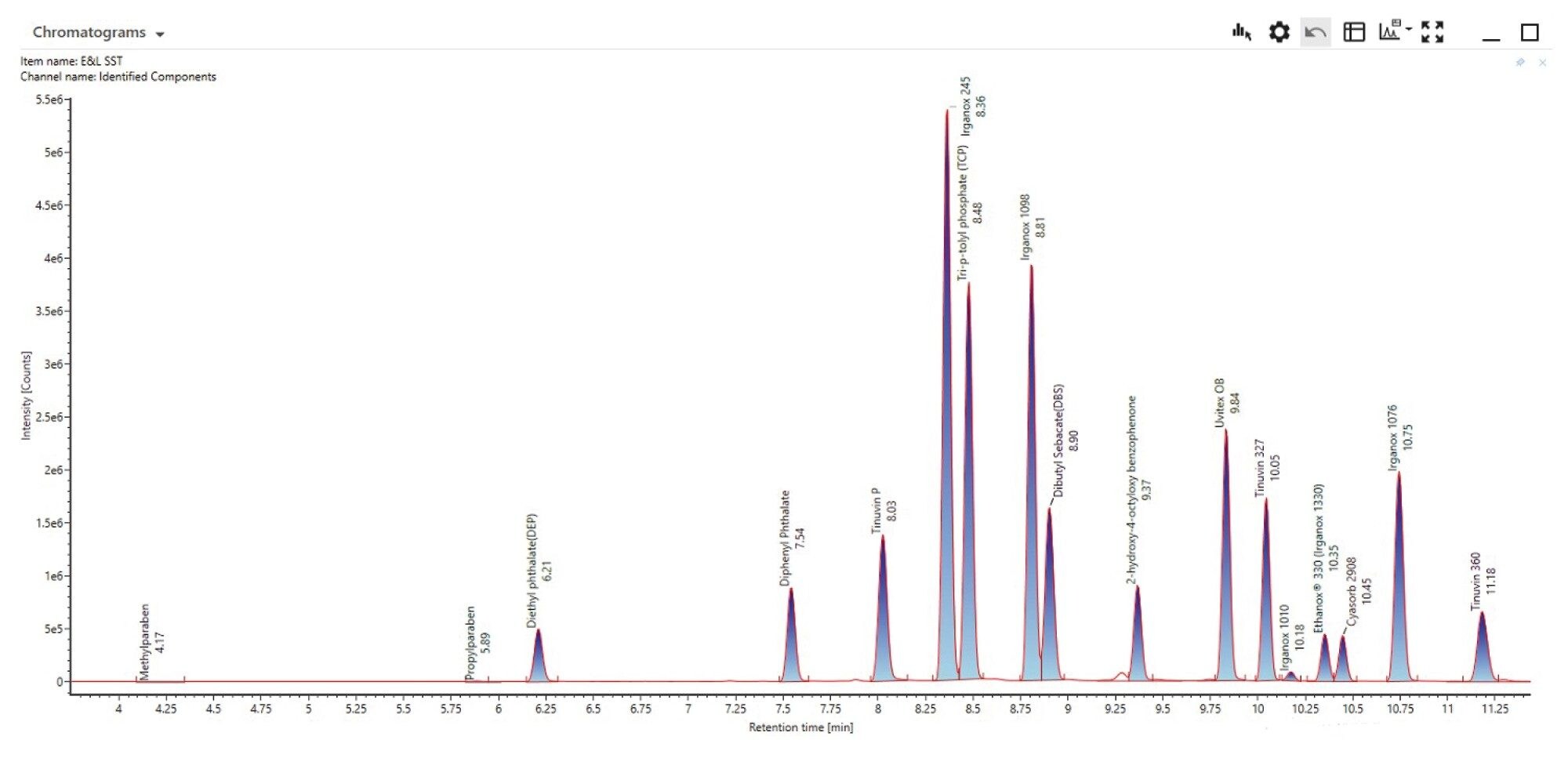
Prior to analysis of the e-liquid sample a standard mix of eighteen common polymer additives (Extractables and Leachables Screening Standard SKU 186008063) was analyzed in both positive and negative mode to serve as a system suitability check.
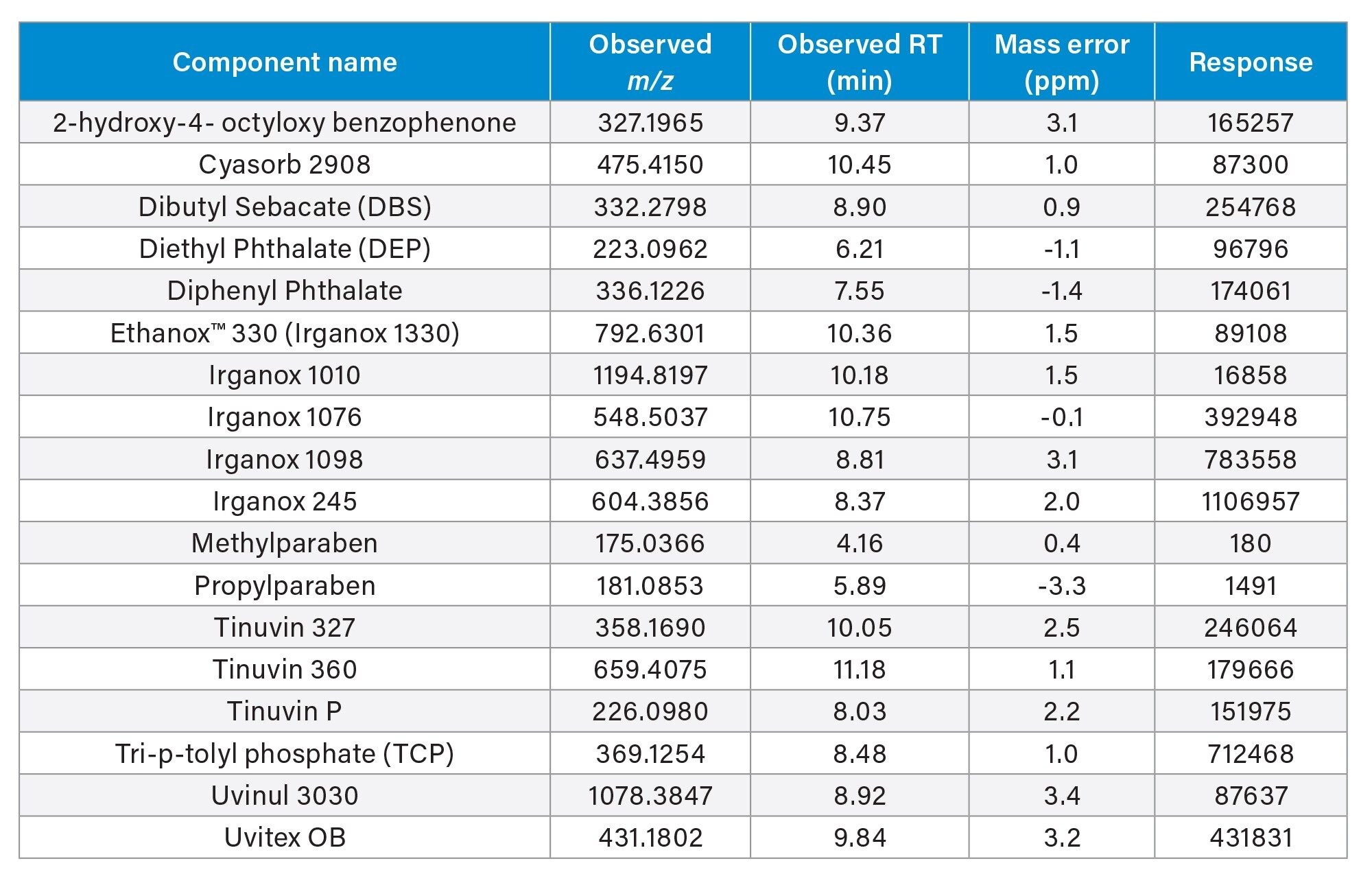
Screening against the E and L library resulted in all eighteen components were detected with mass accuracy results ranging between -3.3 and 3.4 ppm error in positive mode (Figure 2)/(Table 1).
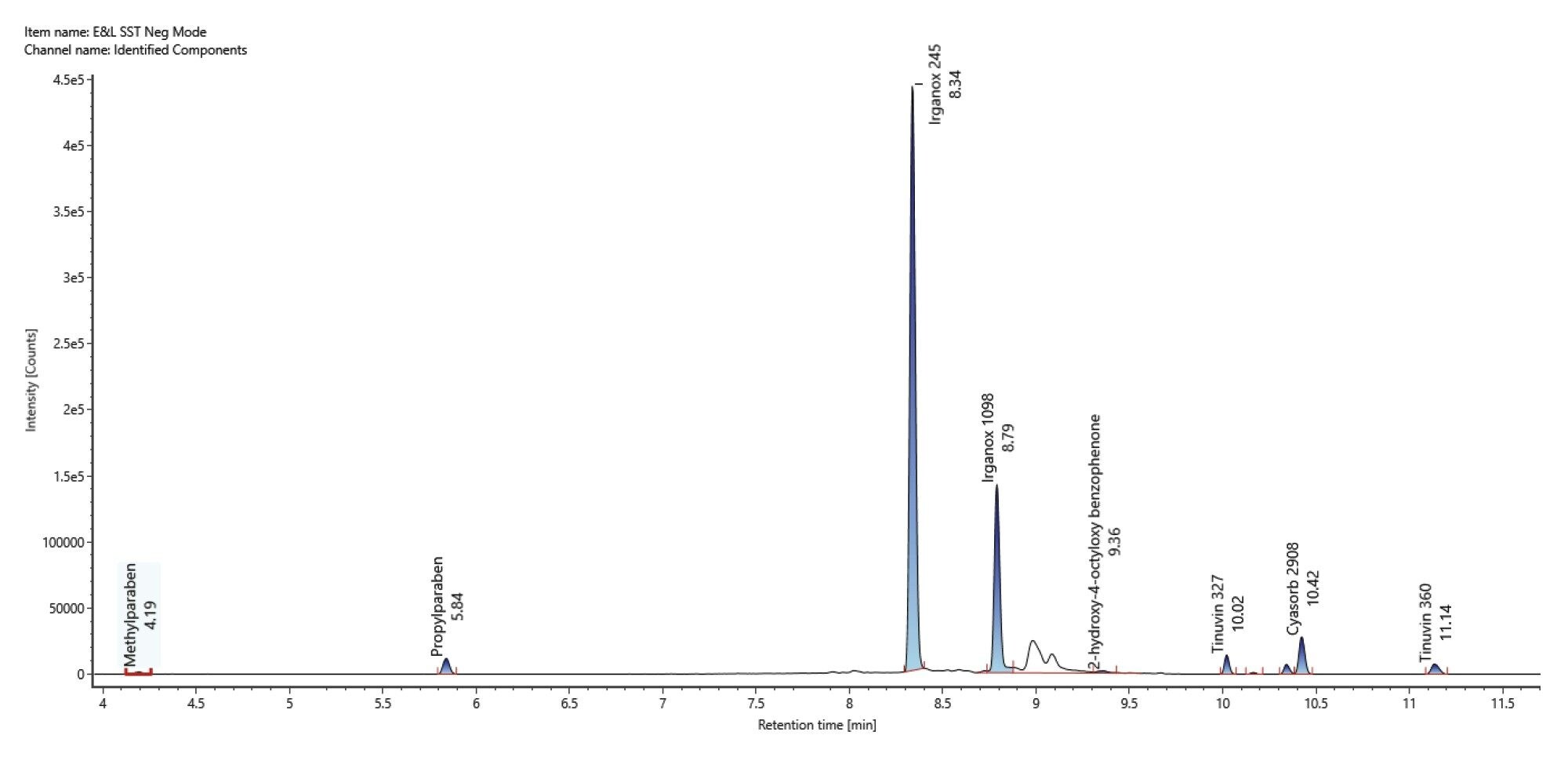
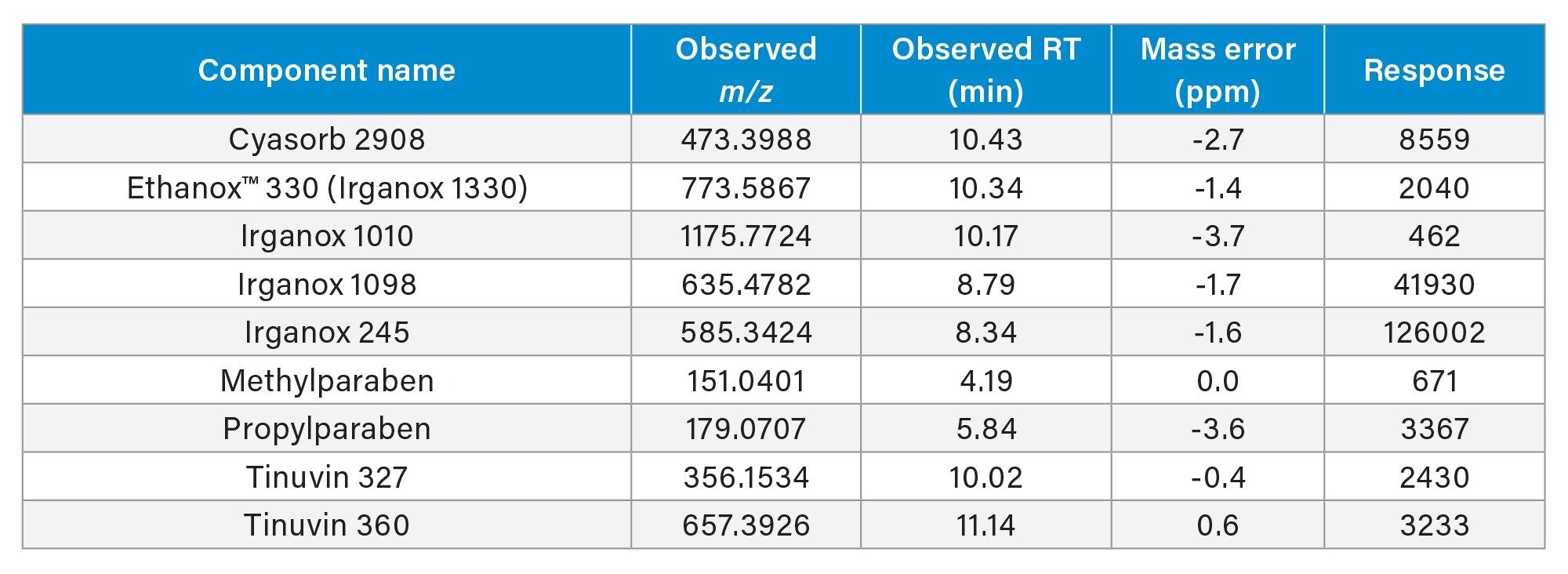
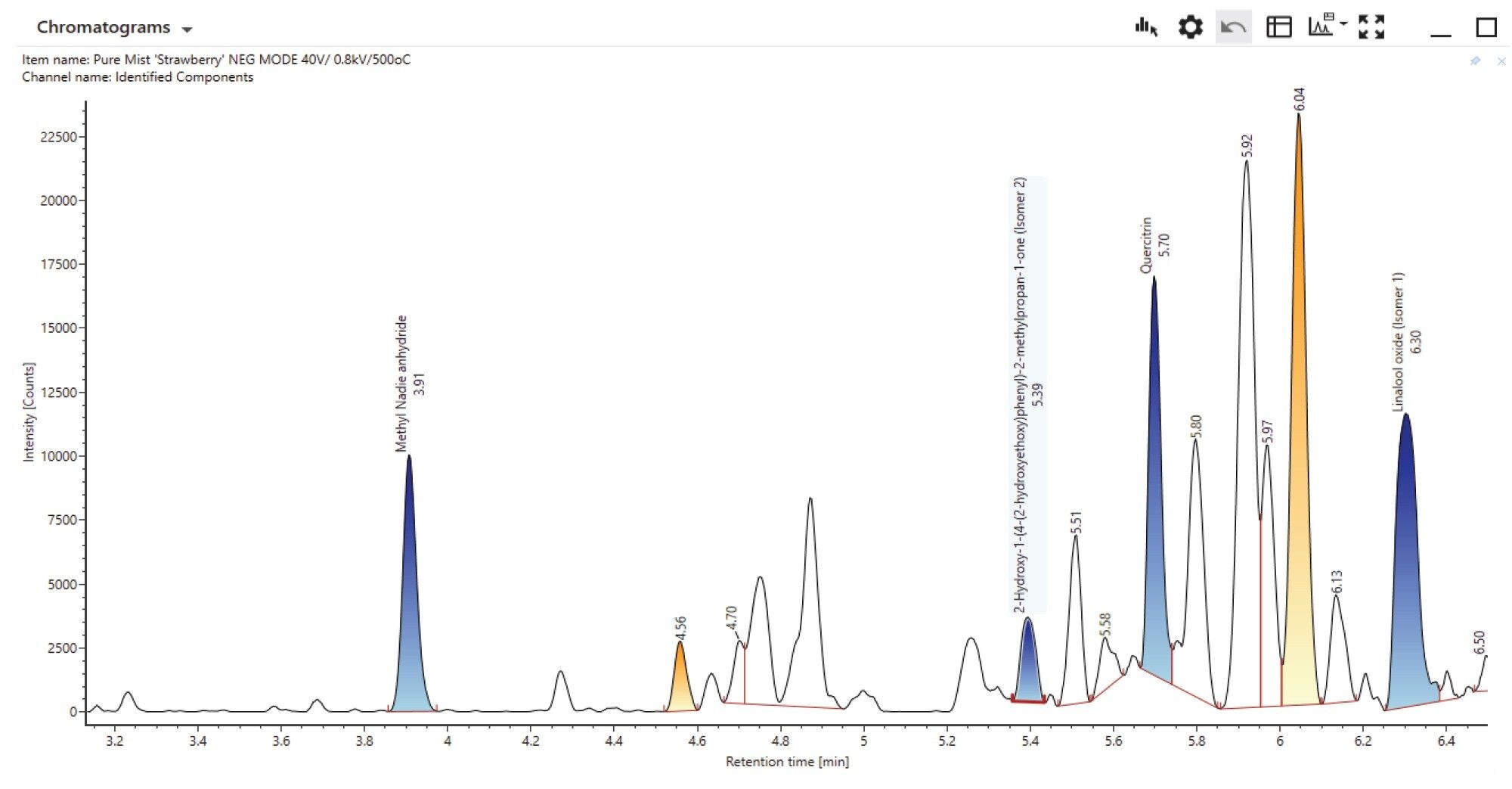
In negative mode 9 compounds were detected and identified with a ppm mass error range of -3.7 and 0.6 (Figure 3)/(Table 2).
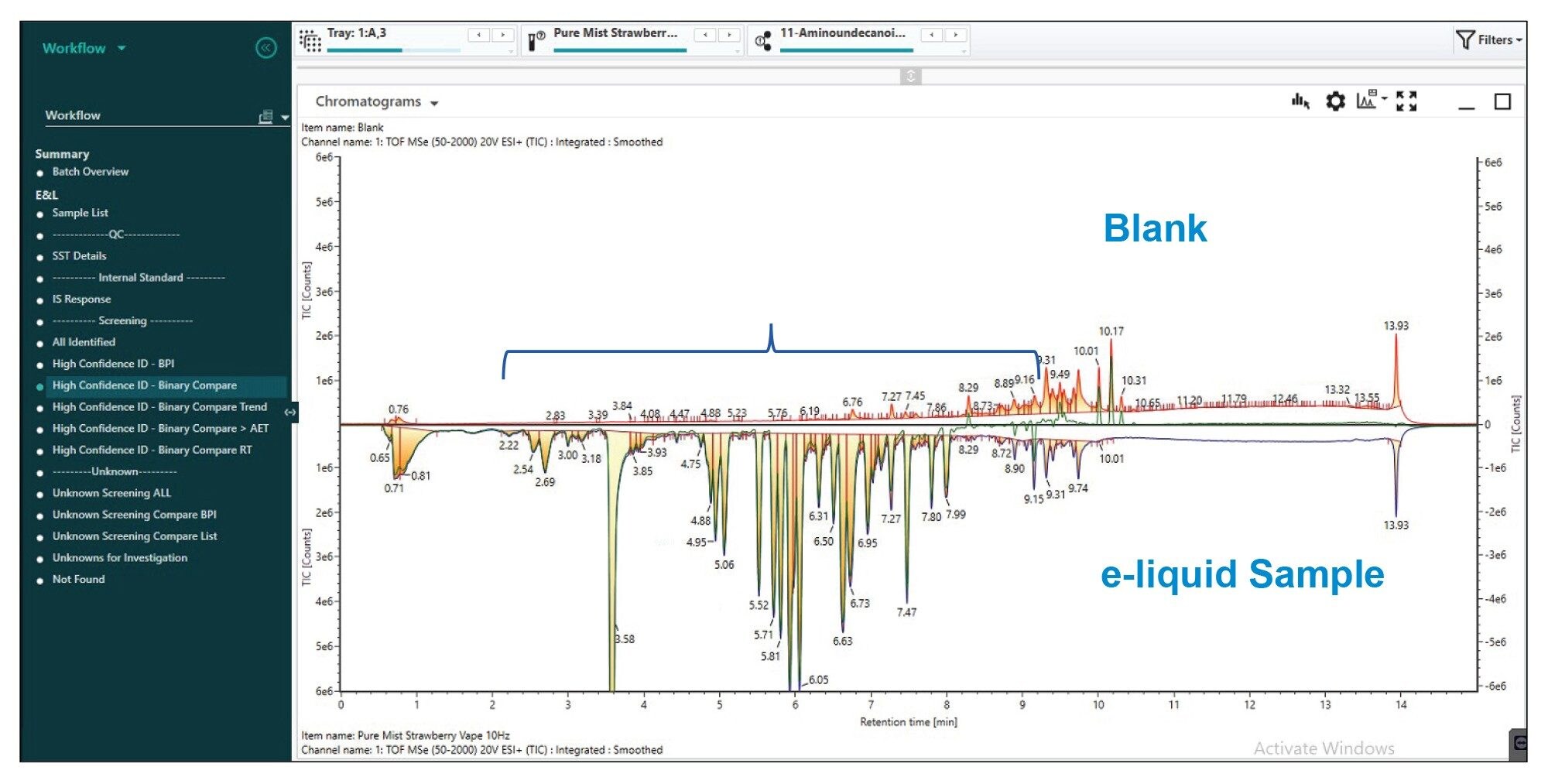
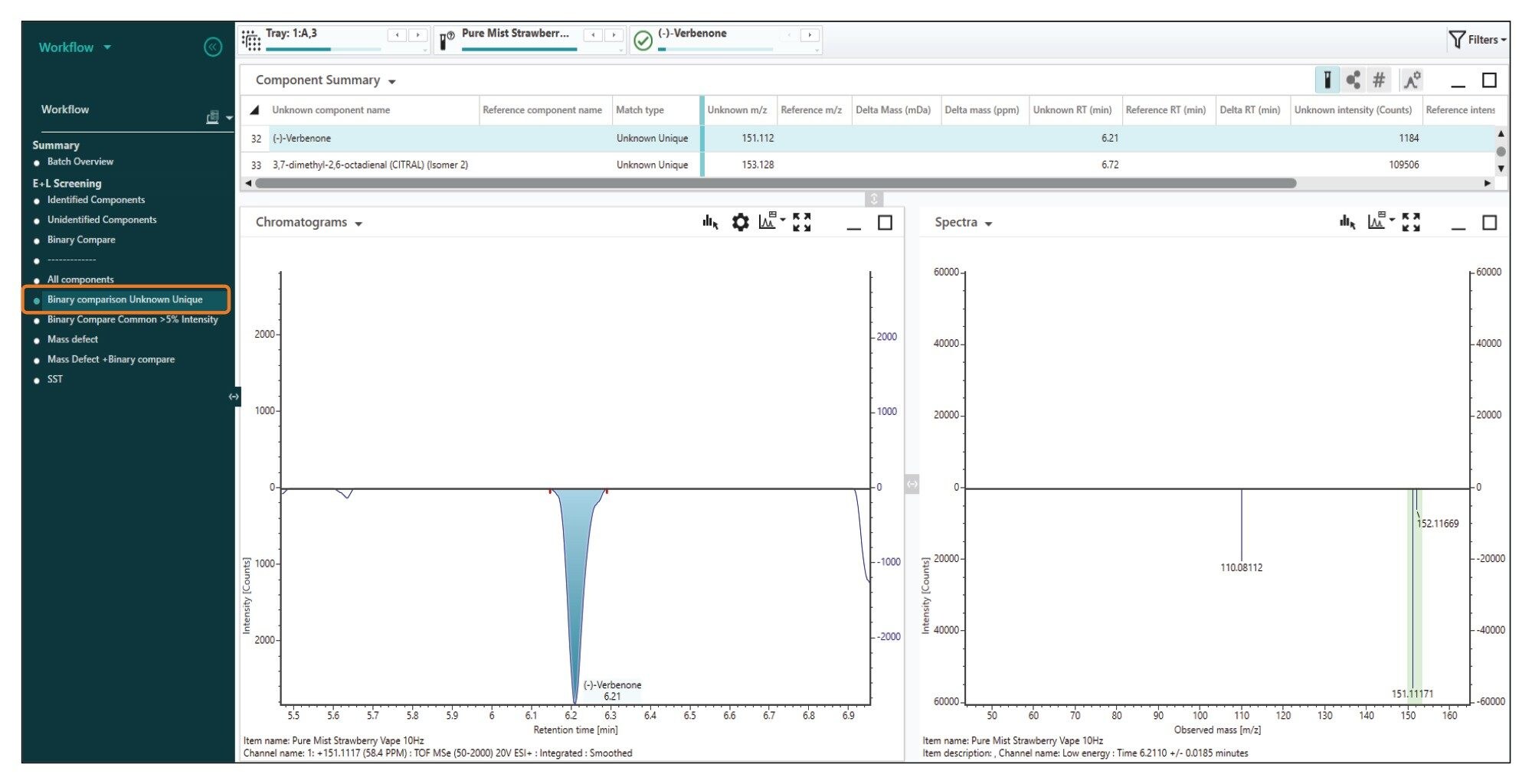
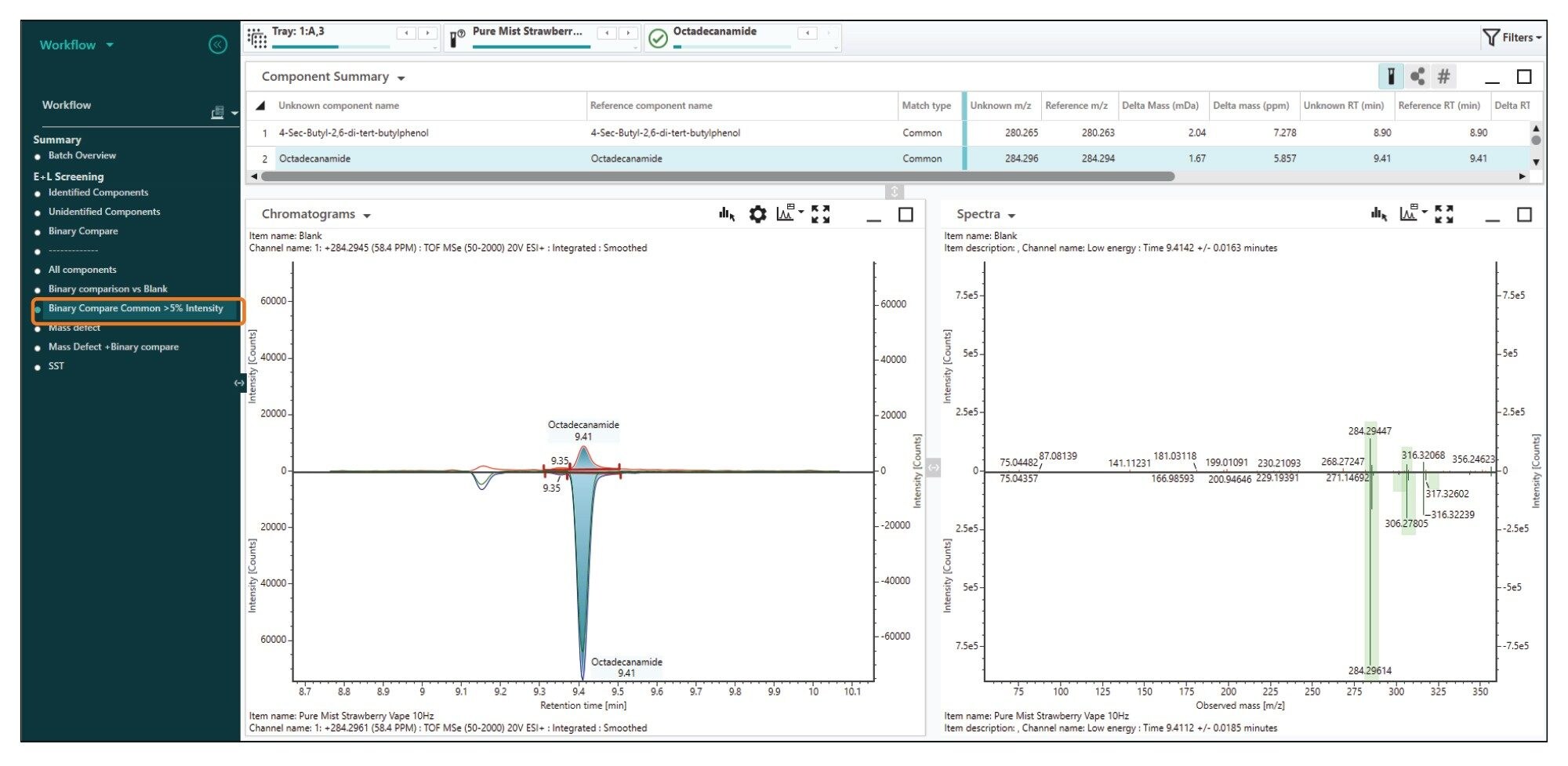
Following the analysis of a mobile phase blanks, the e-liquid diluted sample was analyzed (n=5). Initial processing automatically screened the acquired data against Waters E and L library. Due to the complexity of the data with some manual interrogation required to remove false assignments. Additionally, a blank was processed as a reference spectrum to use in the ‘Binary Compare’ feature which allowed a visual appraisal of TIC (Total Ion Count) of the sample compared to the blank to ascertain which compounds are significant components of the sample (Figure 4). Filters within the workflow allowed the identification of compounds unique to the sample i.e. ‘Unknown Unique’ (Figure 5).
To avoid excluding compounds that might be present in small quantities in the blank, another filter was used. Components that are at least five times more abundant in samples than in blanks were retained, (Figure 6). The filter was set to include both these criteria to ensure all significant components in the sample were captured.
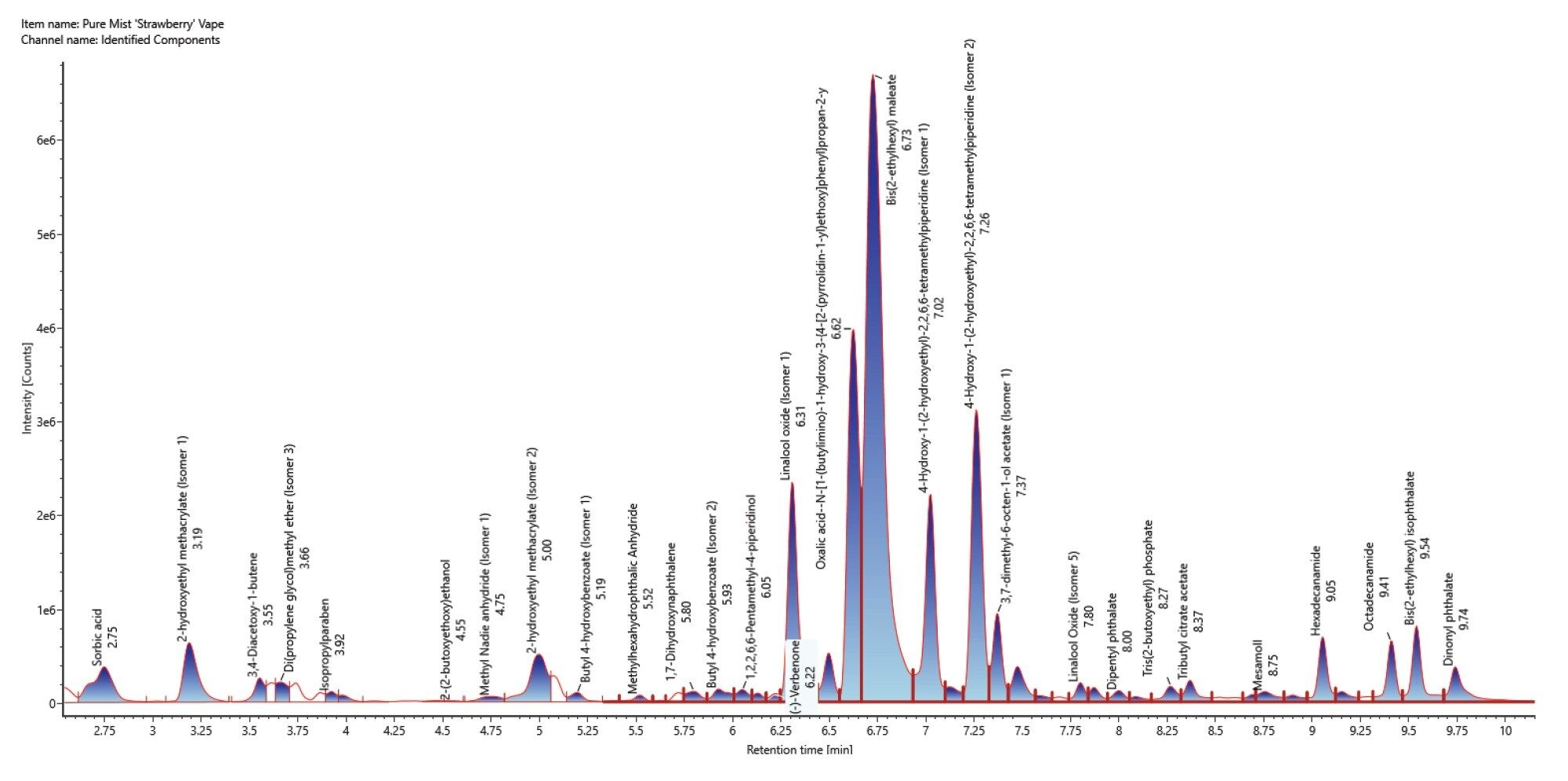
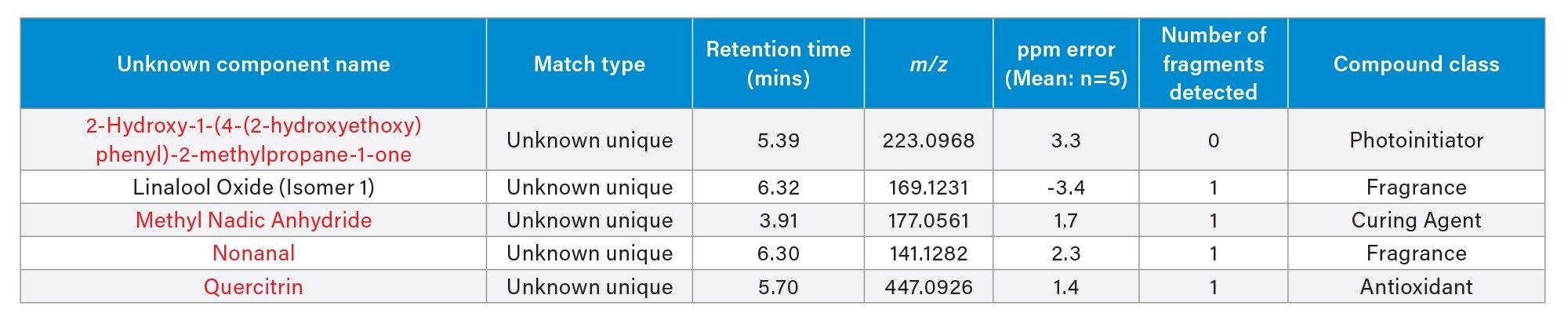
Application of the processing parameters and filters on the positive electrospray mode resulted in thirty-three components confidently identified (Figure 7)/(Table 3) with a mass error ranging between -3.4 and 4.4 ppm. Carbonyl containing compounds are highlighted in red.
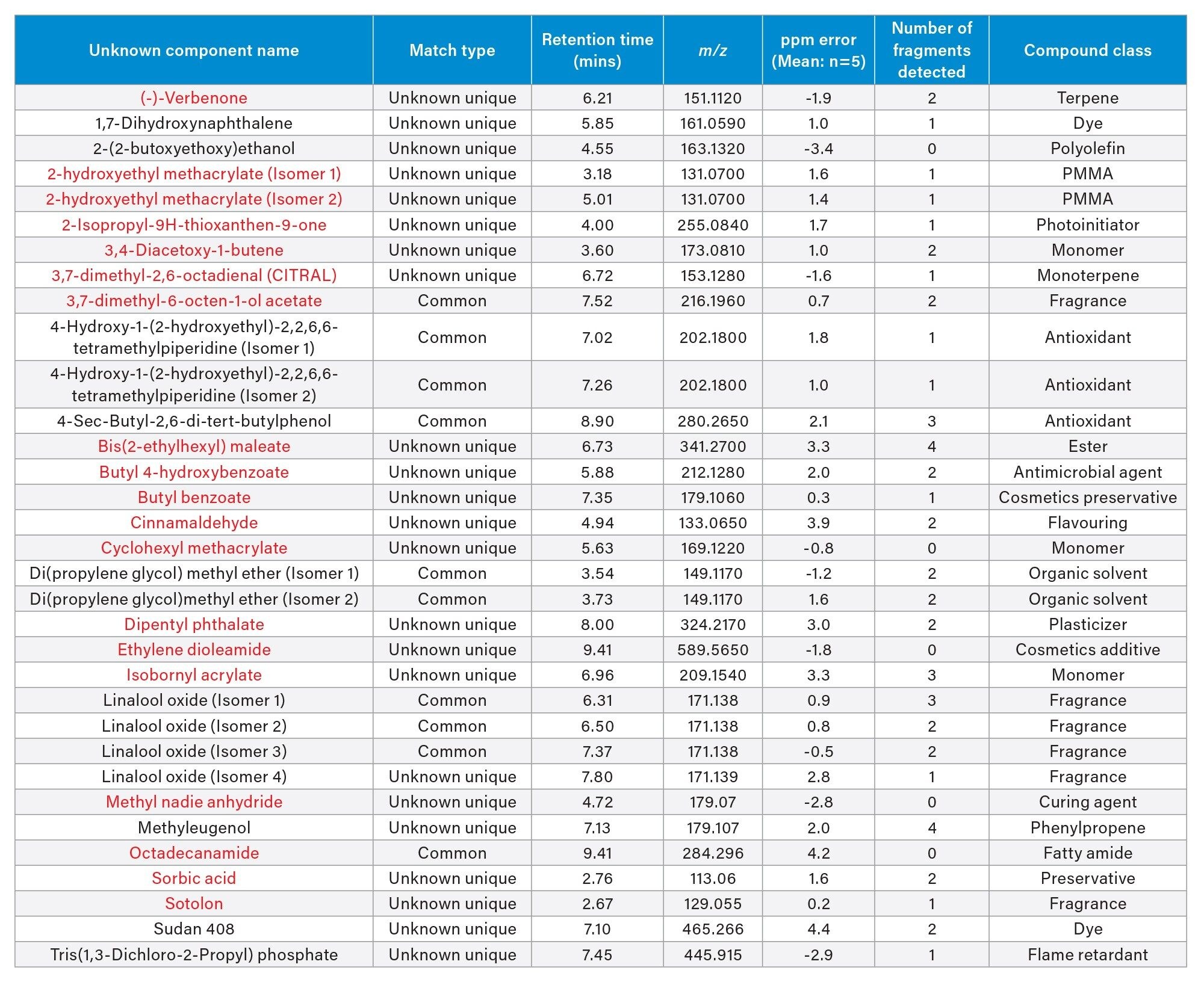
One component identified was cinnamaldehyde-an aromatic aldehyde which is a primary constituent of cinnamon flavorings. While considered safe for ingestion the health impact of cinnamaldehyde and its thermal degradants post heating in an e-cigarette device are unclear. Unsaturated aldehydes such as cinnamaldehyde can degrade upon heating due to double bond breakage leading to the formation of formaldehyde and acetaldehyde.9 Analysis using negative mode electrospray applying the same filter criteria identified five compounds from the screening library (Figure 8)/(Table 4). Again, compounds containing carbonyl groups have been highlighted in red. Of the five compounds detected only ‘Linalool Oxide’ was detected in positive mode.
Discovery Workflow
A significant advantage of routinely acquiring HRMS data is the ability to collect full scan data without compromising sensitivity. In addition, accurate mass data combined with fragment information can enable the tentative assignment of unknown peaks detected within an analysis.
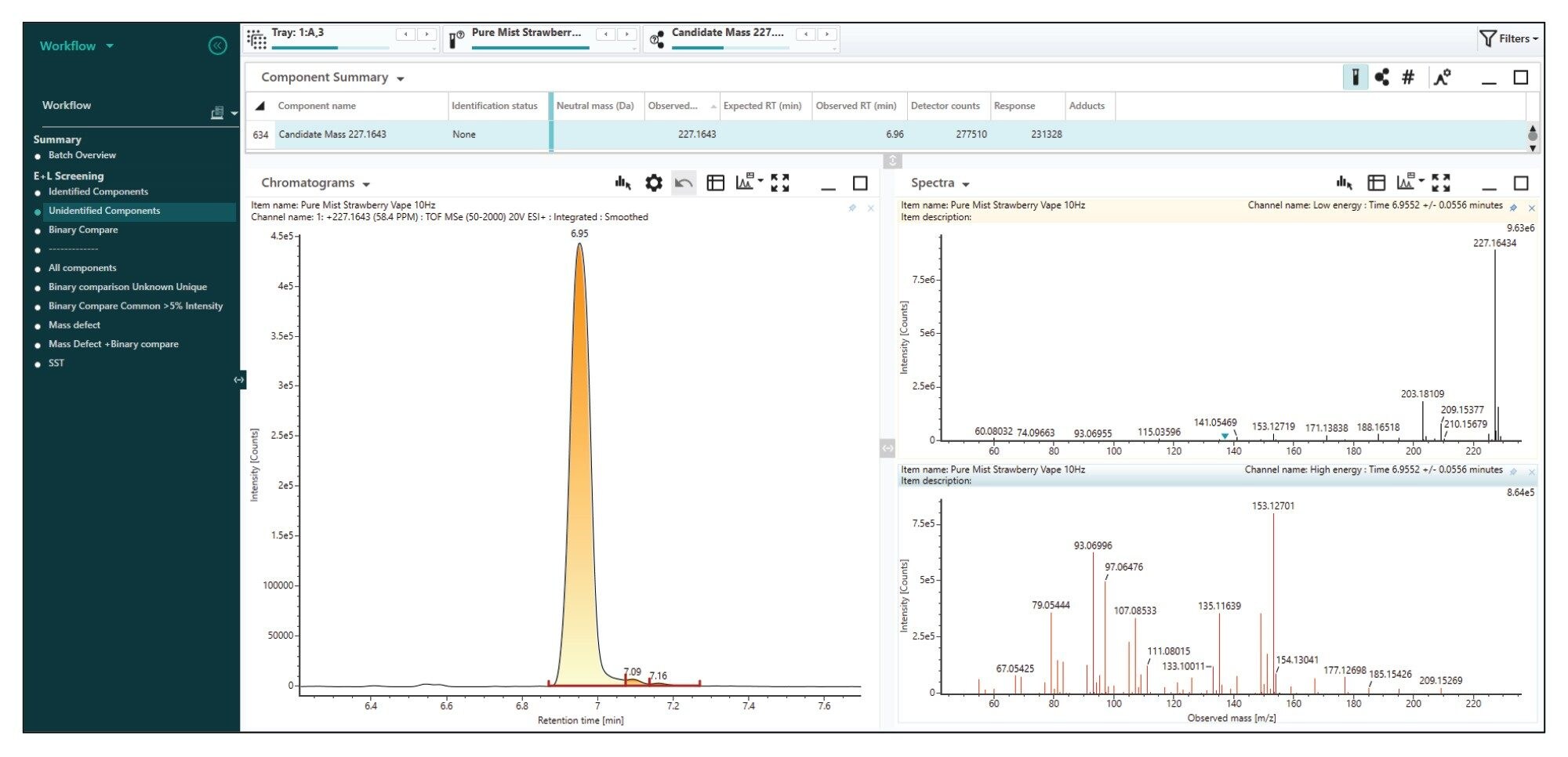
A standard feature within UNIFI there is the ‘Elucidation Toolset’ that facilitates the identification of unknown compounds. The feature enables compound identification by searching online libraries based on elemental composition with structural elucidation using ‘Fragment Match’.
As an example, within the positive mode analysis in the ‘Unknown Components’ workflow, a significant unknown peak at 6.95 minutes with a mass of 227.1643 m/z (Figure 9). This component was not identified in the screening workflow as it is not present in this particular library doesn’t contain an assignment. The elucidation functionality allows generating a molecular formula based on the isotopic pattern and expanding the screening search of the un-assigned component in web based or open-source libraries such as PubChem, ChemSpider.10,11
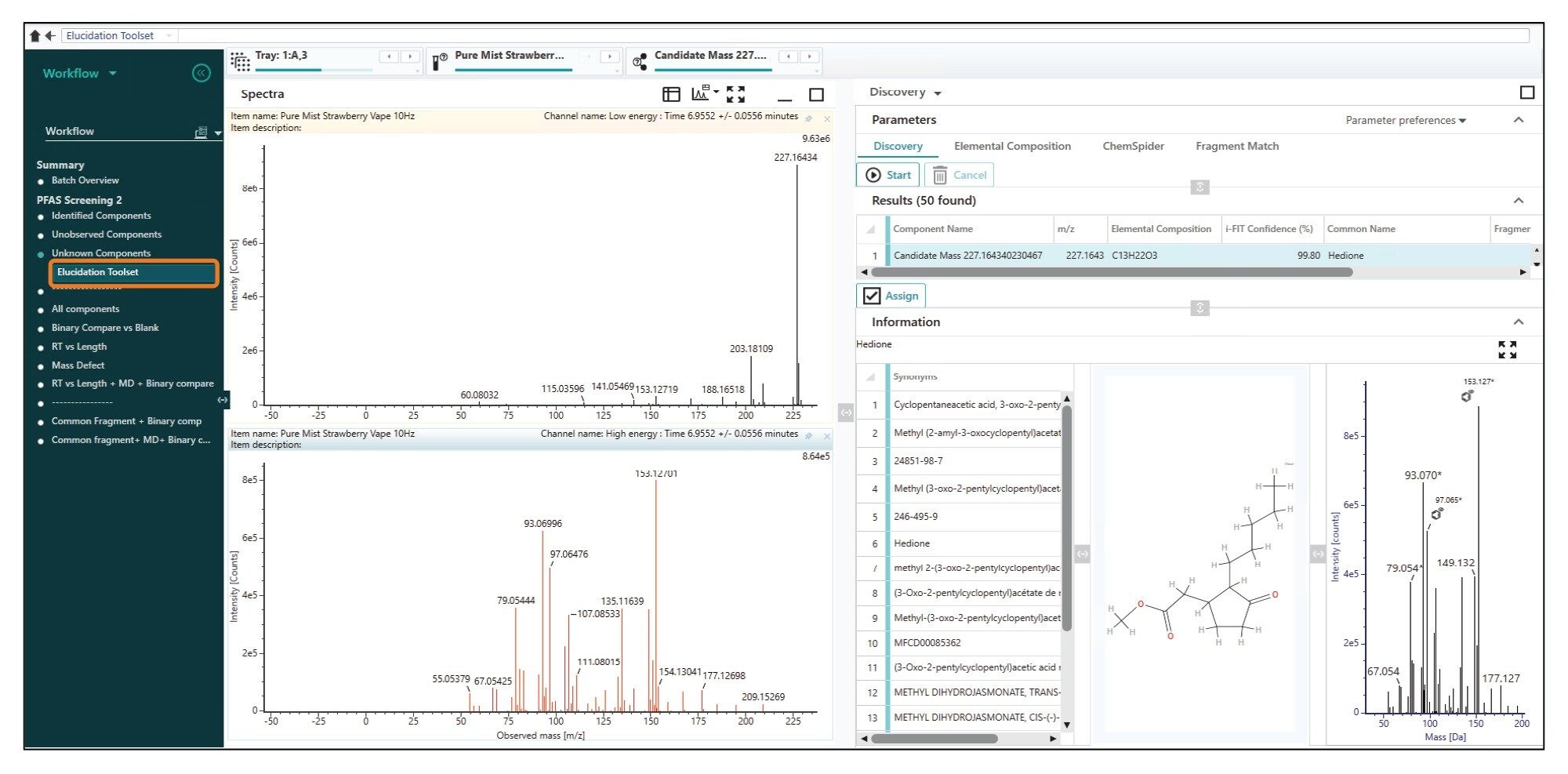
Based on isotopic profile, the Elucidation Toolset returned a potential elemental formula of C13H22O3. Along with fragment information, when screened against online libraries, a number of potential candidates were suggested for the unknown. The i-Fit confidence score assigned by UNIFI is an indication that both the measured m/z and the isotopic pattern are close to the theoretical value of C13H22O3. The higher the i-FIT score the closer the difference between theoretical and experimental values are. From here, a candidate mass with an i-Fit confidence of 99.80% was ‘Hedione’ (Figure 10), a jasmine-like smelling compound used to enhance aroma. This represented a plausible identification and was added to the screening library for future analysis.
Conclusion
With concerns around the quality control and potential health impact of ‘e-liquids’, there is an increasing need for manufacturers and laboratory services to get the necessary instrumentation in place to provide high quality characterization of these products.
The ACQUITY RDa coupled to the ACQUITY I-Class PLUS has demonstrated the ability to provide fit for purpose information for screening and discovery workflows in an easy to use format for deployment in routine analytical labs.
In positive electrospray mode thirty three compounds were identified from the E and L screening libraries, with nineteen of those compounds identified containing a carbonyl group which can then be monitored for potential toxicity. This raises concerns about the contents of ‘e-liquids’ due to the potential toxicity of inhaled carbonyl compounds. In negative electrospray mode five compounds were detected with four containing a carbonyl group negative mode.
The discovery workflow pointed out the presence of assignment of ‘hedione’. Hedione is used in cosmetics or food flavoring, not present in the E and L library. Screening online libraries was essential for the assignment of unknown compounds. A comparison of the m/z, retention time, and fragmentation of hedione with a synthetic standard if required for full identification. Combined with the compliant-ready UNIFI HRMS screening software as part of waters_connect, the ACQUITY RDa provides a simple solution to acquiring high quality data for a wide range of analytical expertise for e-liquids in a regulated environment.
References
- McDermott MS, East KA, Brose LS, McNeill A , Hitchman SC, Partos TR. “The Effectiveness of Using E-Cigarettes for Quitting Smoking Compared to Other Cessation Methods Among Adults in the United Kingdom”. Society for the Study of Addiction. 2021 Oct;116(10):2825–2836. doi: 10.1111/add.15474. Epub 2021 May 4.
- Mishra A, Chaturvedi P, Datta S, Sinukumur S, Joshi P, Garg A. “Harmful Effects of Nicotine”. Indian J Paidiatric Oncol. 2015 Jan-Mar;36(!):24–31. doi: 10.4103/0971–5851.151771.
- Gholap VV, Kosmider L, Halquist MS. “A Standardized Approach to Quantitative Analysis of Nicotine in E-Liquids Based on Peak Purity Criteria Using High-Performance Liquid Chromatography”. Hindawi Journal of Analytical Methods in Chemistry, Volume 2018, Article ID 1720375, 11 pages, https://doi.org/10.1155/2018/1720375.
- Schober W, Szendrea K, Matzea W, et al. Use of Electronic Cigarettes (e-cigarettes) Impairs Indoor Air Quality and Increases FeNO Levels of e-cigarette Consumers. Int J Hyg Environ Health. 2013;217:628–637. doi:10.1016/j.ijheh.2013.11.003.
- Famele M, Ferranti C, Abenavoli C, Palleschi L, Mancinelli R, Draisci R. “The Chemical Components of Electronic Cigarette Cartridges and Refill Fluids: Review of Analytical Methods.” Nicotine & Tobacco Research, 2015, 271–279,doi:10.1093/ntr/ntu197. Advance Access publication September 25, 2014.
- Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. Carbonyl Compounds Generated from Electronic Cigarettes. Int J Environ Res Public Health. 2014 Oct 28;11(11):11192–11200. doi: 10.3390/ijerph111111192.
- Menicagli R, Marotta O, Serra R. “Free Radical Production in the Smoking of E-Cigarettes and Their Possible Effects in Human Health”. Int J Prev Med. 2020 Apr 30;11:53. doi: 10.4103/ijpvm.IJPVM_424_19.
- A Review of Waters Hybrid Particle Technology. Part 2: Ethylene Bridged [BEH Technology™] Hybrids and Their Use in Liquid Chromatography.
- Nistoriak MA, Kilfoil PJ, Lorkiewicz PK, Ramesh B, Kuehl PJ, McDonald J, Bhatnagar A, and Conklin DJ. “ Comparative Effects of Parent and Heated Cinnemaldehyde on the Function of Human iPSC-Derived Cardiac Myocytes.”Toxicol In Vitro 2019 Dec;61: 104648. doi: 10.1016/j.tiv2019.104648.
- https://pubchem.ncbi.nlm.nih.gov.
- https://www.chemspider.com.
720008638, December 2024