
In this application we demonstrate the increased polar retention of CORTECS T3 Columns for the isocratic separation of catechin and its metabolites.
Increase in polar retention compared to traditionally bonded solid-core C18 columns.
Analysis of polar analytes under reversed phase liquid chromatography (RP-LC) conditions can yield poor retention and co-elution of early eluting compounds. This is due to a lack of attractive interactions between the polar analytes and the hydrophobic stationary phase. However, RP-LC columns specifically designed to facilitate polar compound analysis can overcome these limitations. Traditional C18 bonded phases on solid-core particles typically employ particles having pore diameters ranging from 80–100 Å, have relatively high bonding coverages, and are endcapped. These factors make traditional C18 bonded phases very useful for hydrophobic analytes, but less retentive for polar molecules.
CORTECS T3 Columns provide reproducible, superior retention for polar analytes compared to other solid-core columns containing traditional C18 bonded phases. While CORTECS T3 is a C18 bonded phase, it has two features that enhance polar molecule retention compared to traditional C18 bonded phases. The first feature is improved access of polar analytes to the silanol groups of the underlying base particle. This gives better polar interactions and thereby increased polar retention. Such access is enabled using a lower C18 bonding density. The second feature is improved resistance to dewetting.1,2 Originally called "phase collapse", dewetting happens with low or no organic (strong) solvent mobile phase compositions when the eluent is forced out of the stationary-phase pores. This causes a loss of interactions between the stationary phase and the analytes, as the analytes no longer have access to the interior of the particles, producing reduced retention and often peak distortion as well. Resistance to dewetting is made possible by using particles with a wider pore size and is also assisted by the lower bonding density.1
This combination of design features allows reproducible use of eluents containing low or no organic solvent, giving maximum retention. In this application we demonstrate the increased polar retention of CORTECS T3 Columns for the isocratic separation of catechin and its metabolites.
|
LC system |
ACQUITY UPLC H-Class with Column Manager |
|
Columns |
CORTECS UPLC T3, 1.6 μm, 2.1 x 100 mm (p/n: 176003894) CORTECS UPLC C18, 1.6 μm, 2.1 x 100 mm (p/n: 186007095) CORTECS T3, 2.7 μm, 2.1 x 100 mm (p/n: 186008484) CORTECS C18, 2.7 μm, 2.1 x 100 mm (p/n: 186007367) Phenomenex Kinetex C18, 2.6 μm, 2.1 x 100 mm Phenomenex Kinetex C18, 1.7 μm, 2.1 x 100 mm Advanced Materials Technology HALO C18, 2.7 μm, 2.1 x 100 mm Thermo Scientific Accucore C18, 2.6 μm, 2.1 x 100 mm |
|
Mobile phase |
95:5 (water:acetonitrile with 0.1% formic acid) |
|
Flow rate |
0.6 mL/min |
|
Column temperature |
30 °C |
|
Detection (UV) |
280 nm |
|
Injection volume |
1.0 μL |
|
Data management |
Empower 3 CDS |
A sample mix containing the following compounds at the described concentrations was created using water.
Catechin is a natural antioxidant and is a constituent of many different plants including certain types of tea. The structures of catechin and related metabolites (Figure 1) show considerable hydroxyl functionality. These compounds are polar in nature and are not retained well under typical reversed-phase liquid chromatography conditions. Specifically, using too much strong mobile-phase solvent, such as acetonitrile or methanol, will cause the compounds to elute off the column too quickly.
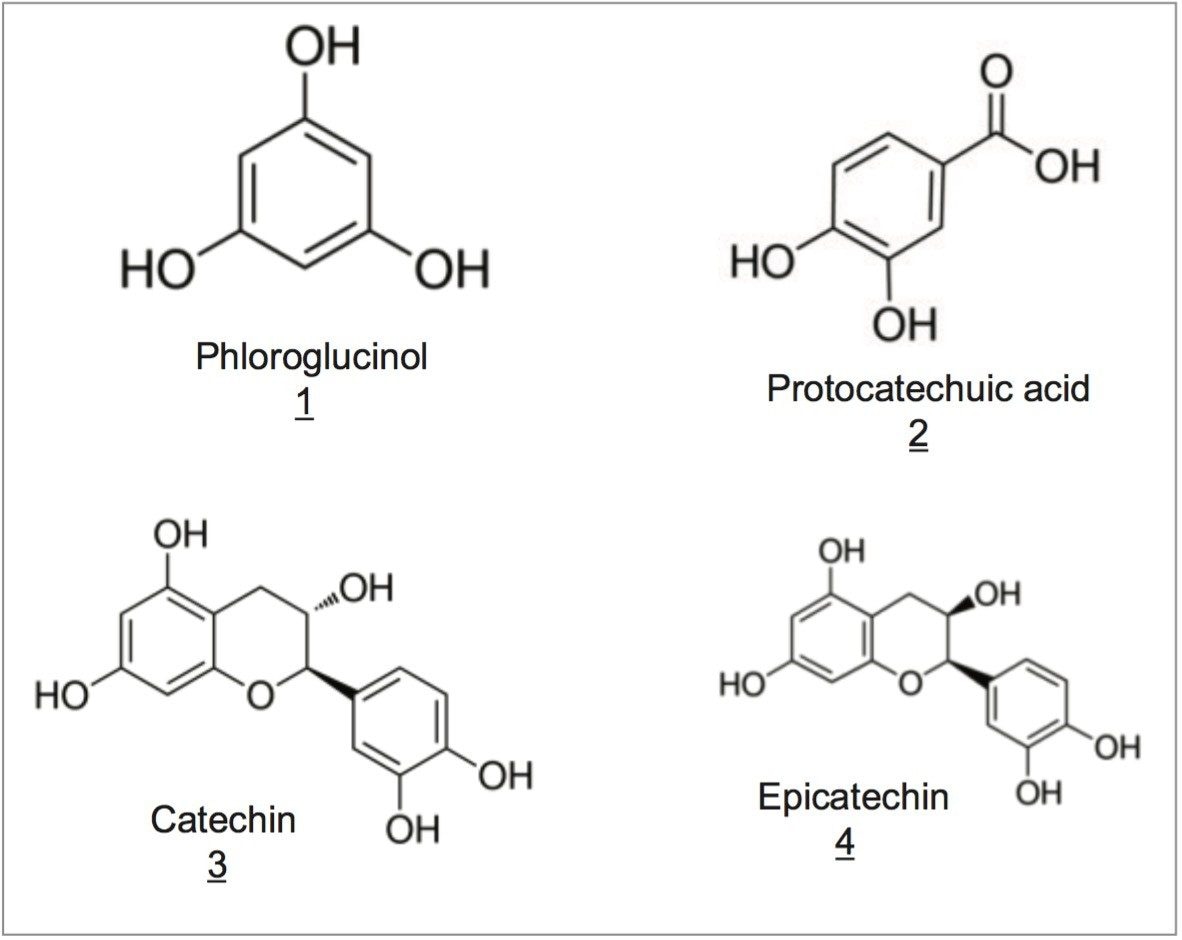
Using an isocratic method with a mobile phase consisting of 5% acetonitrile in water, these compounds were separated on several solid-core C18 columns, including a CORTECS T3 Column. Table 1 shows the columns tested as well as the particle sizes, pore sizes, and C18 surface concentrations of each, where available.
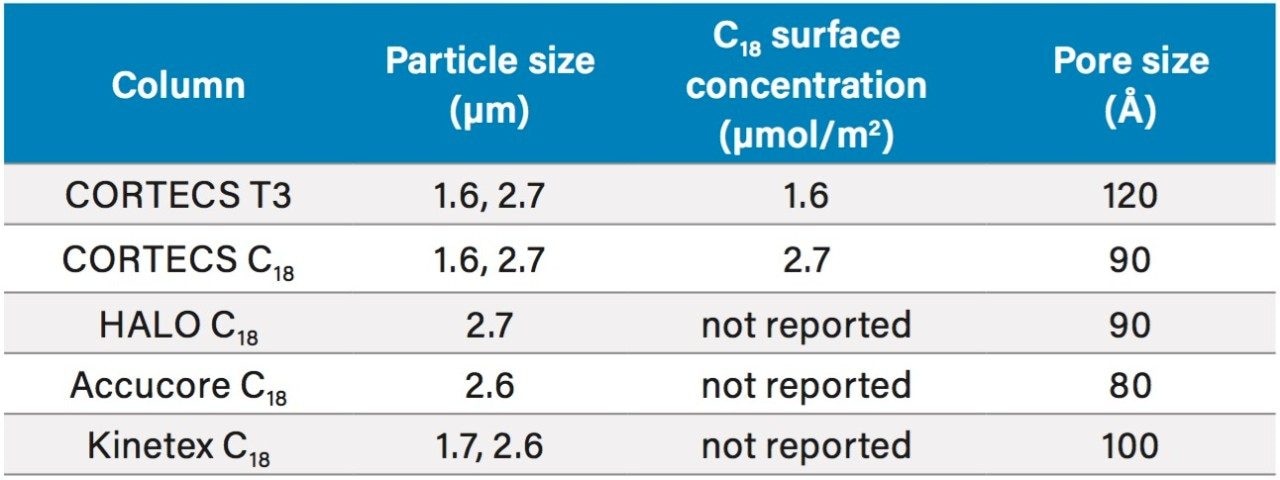
CORTECS T3 has the largest pore size of the columns tested, with Kinetex C18 being second. Additionally, the C18 surface concentration of CORTECS T3 is much lower than that of CORTECS C18. The design features of CORTECS T3 allow for more retention of polar analytes, especially when using low percentages of organic solvent in the mobile phase. Figure 2 shows the separation of catechin and related metabolites on five 2.1 x 100 mm, 2.6–2.7 µm solid-core columns.
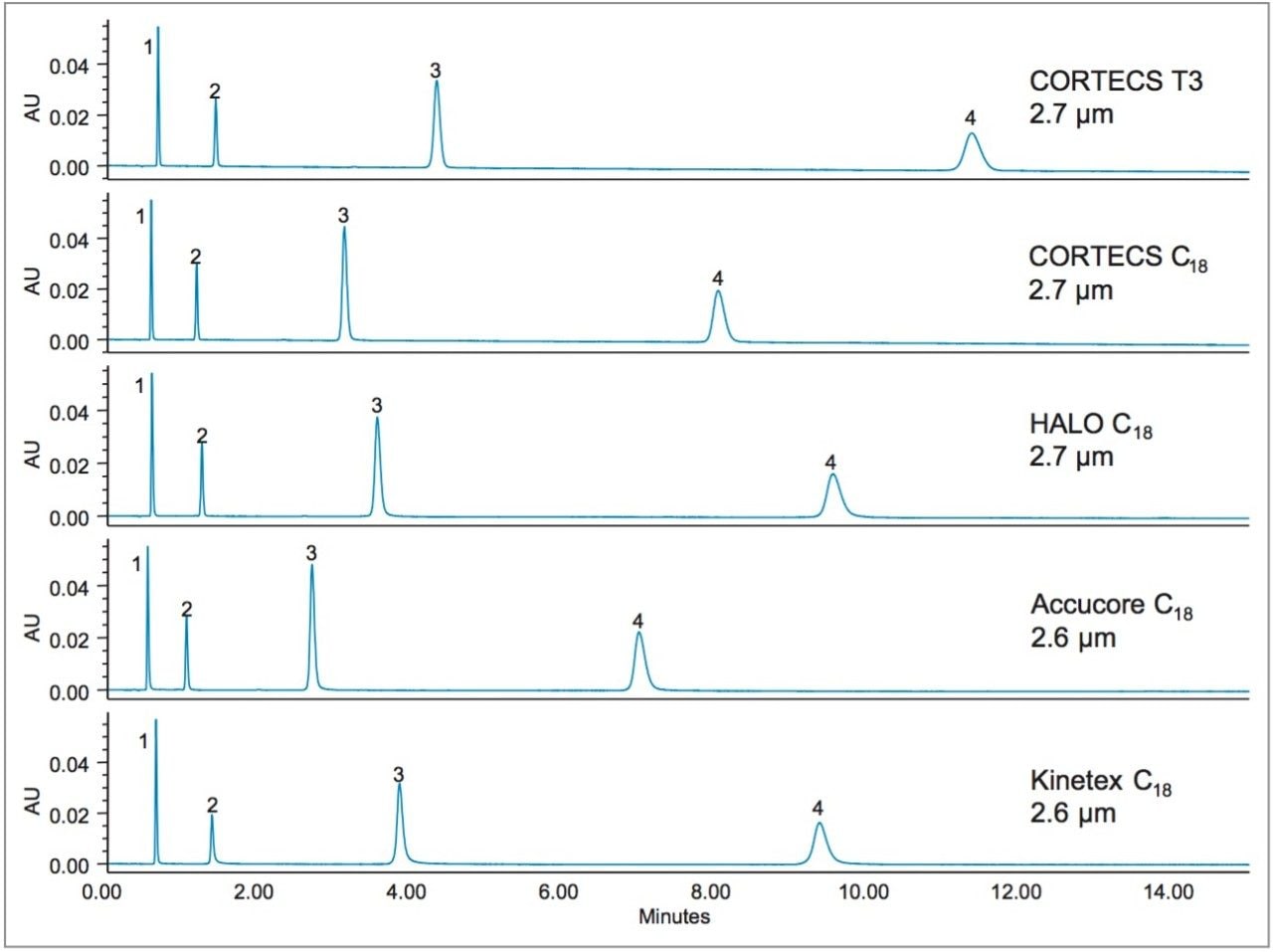
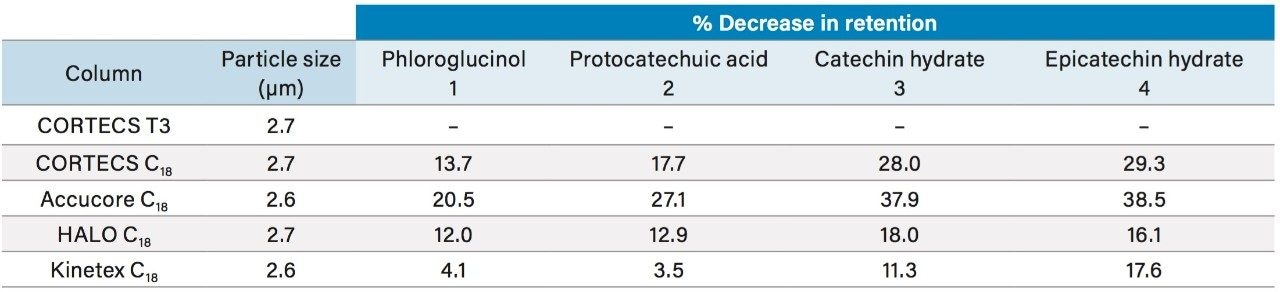
The chromatograms demonstrate that the CORTECS T3 Column retains these compounds more than any of the other columns tested. The CORTECS T3 Column shows a 16% increase in retention time for the latest eluting peak, epicatechin, compared to the next most retentive column, HALO C18. Additionally, for the earliest eluting peak, phloroglucinol, the CORTECS T3 Column achieves a 4% increase in retention time over the next most retentive column, Kinetex C18. The decreases in retention times for each column relative to the CORTECS T3 Column are shown in Table 2.
The benefits of the CORTECS T3 stationary phase are also available in a smaller particle size, allowing not only increased retention, but also increased column efficiency. Figure 3 shows the separation of catechin and related metabolites on 2.1 x 100 mm, 1.6–1.7 µm particle size columns. Only three columns were tested using these dimensions as some solid-core technologies are not available in sub-2-µm particle sizes.
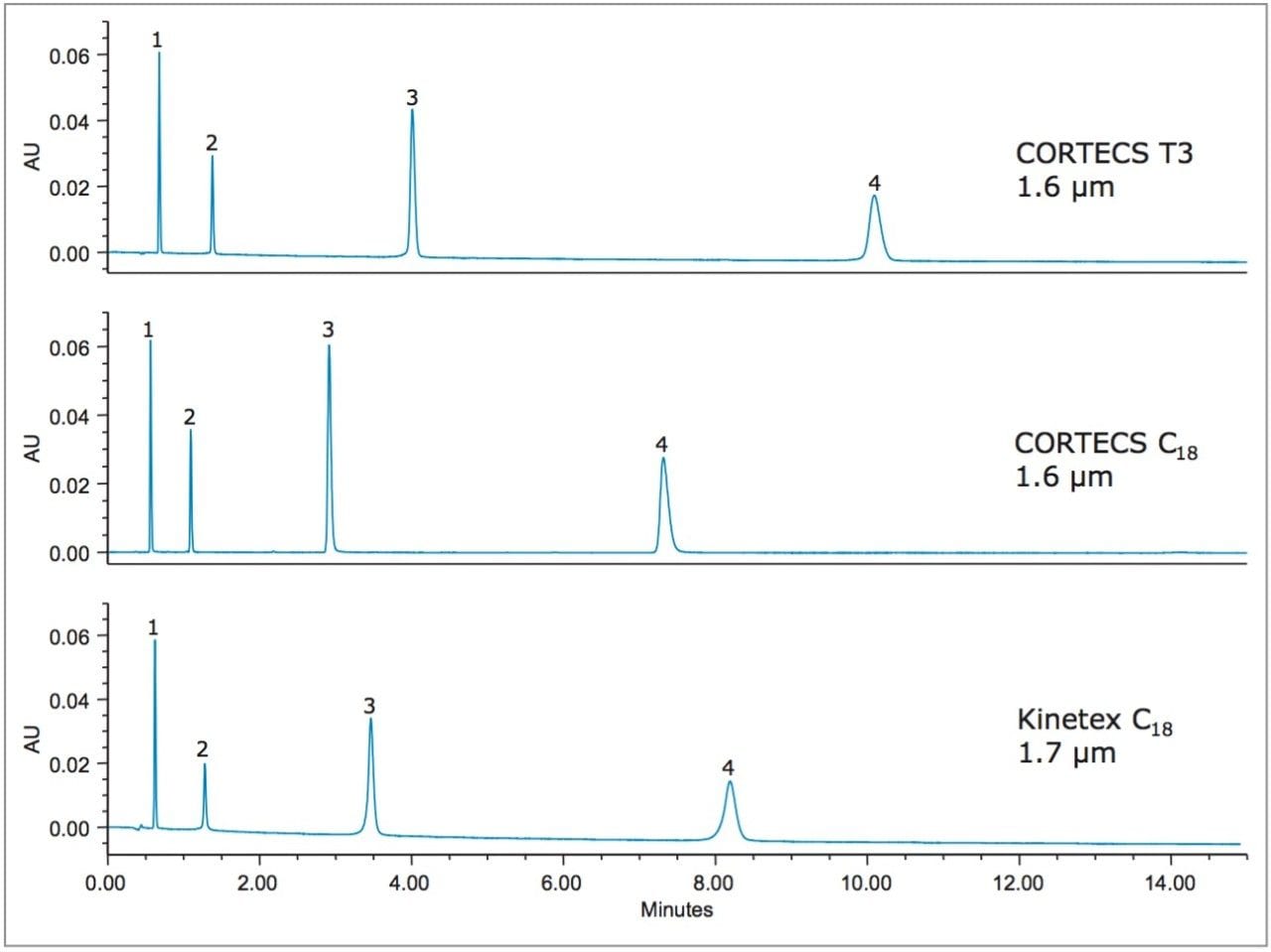
Once again the CORTECS T3 Column gave greater retention for these compounds compared to the other columns tested. As shown in Table 3, the CORTECS T3 Column gave 19% and 8% increases in retention time for the latest and earliest eluting peaks respectively, compared to the next column, Kinetex C18.
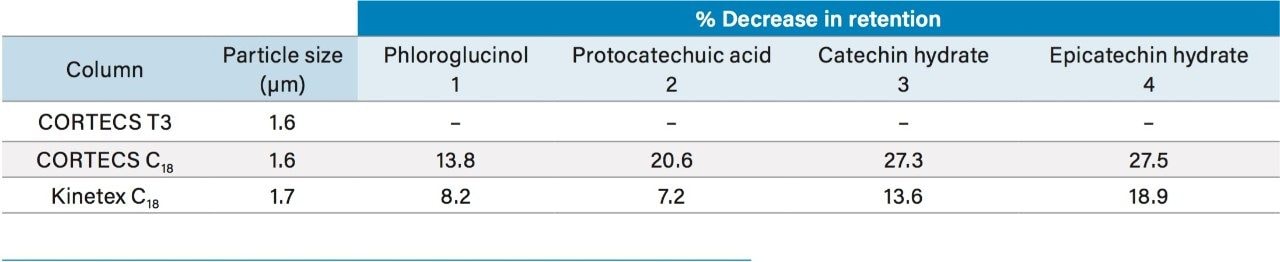
Using CORTECS T3 Columns, an analyst can obtain greater retention for polar analytes compared to other solid-core C18 columns. This may result in better compound characterization with avoidance of peak shape disturbances sometimes observed for early eluting compounds.
Analysis of polar compounds can be challenging in RP-LC. However, CORTECS T3 Columns have been specifically designed to maximize the retention of polar analytes under reversed phase conditions. CORTECS T3, a C18 bonded stationary phase, has a lower C18 surface concentration and an increased pore size compared to traditional C18 bonded phases on solid-core particles. These properties allow polar analytes to better interact with the stationary phase, increasing retention and providing better chromatography for such compounds. In addition, these design features allow for robust operation when using mobile phases containing low concentrations of organic solvents, avoiding issues caused by dewetting.
720005946, March 2017