For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
In this application brief, we show the accuracy and precision possible in the analysis of caffeine using the ACQUITY UPLC System and the Quattro Premier Mass Spectrometer.
Caffeine is a very common analyte within bioanalytical research, due its use as a CYP450 marker and its common application in co-dosing studies. In this application brief, we show the analysis of caffeine in protein precipitated human plasma, performed using the Waters ACQUITY UltraPerformance LC System coupled with the Waters Micromass Quattro Premier Mass Spectrometer. In this work, we have used an external calibration method to highlight the excellent accuracy and precision of this system configuration.
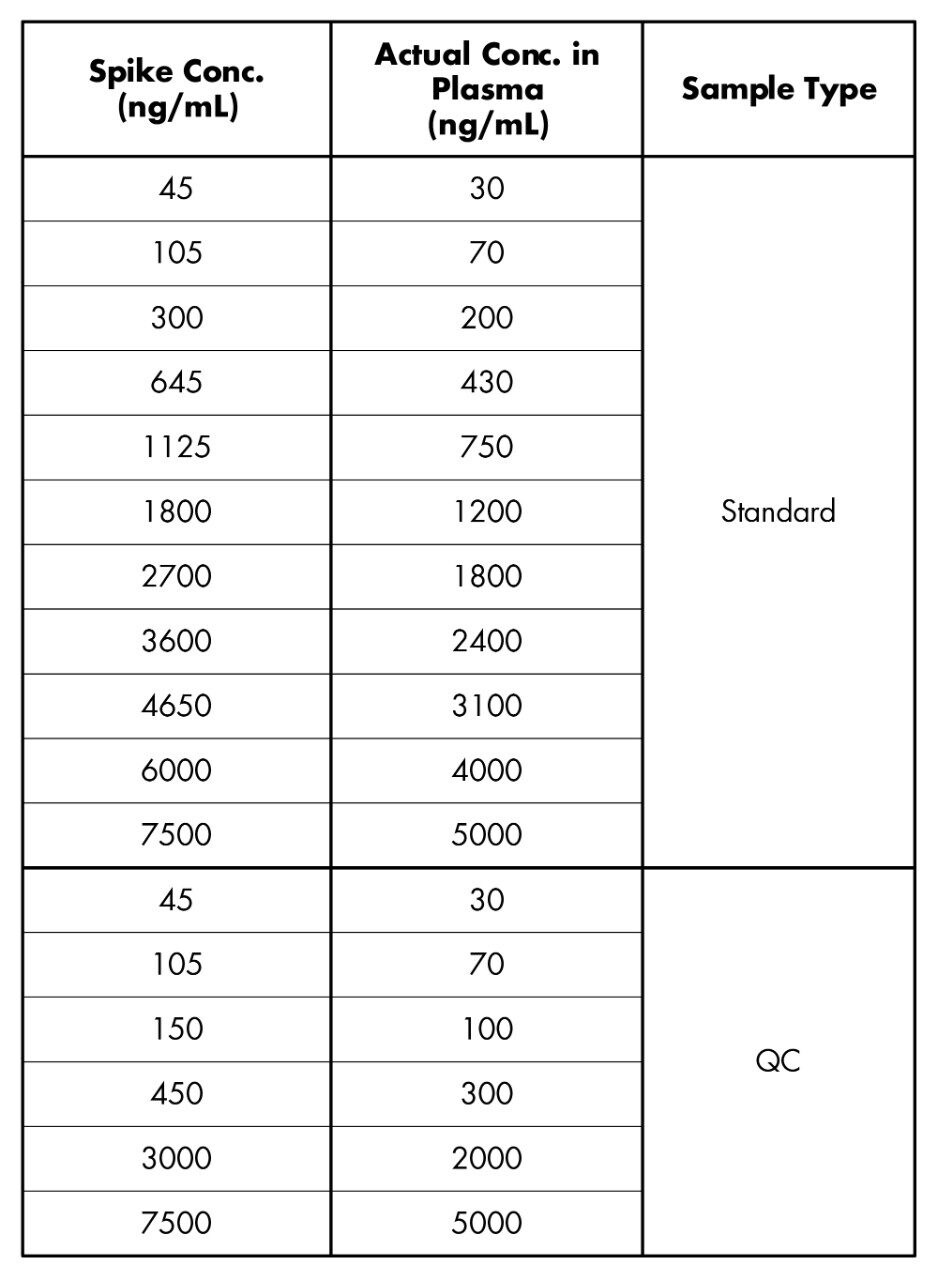
Caffeine spiking solutions were made up at 14 concentrations between 45 and 7500 ng/mL in water as shown in Table 1.
First, 100 μL of spiking solution was added to 200 μL of human plasma. Next, 1.0 mL of acetonitrile was added to perform the protein precipitation, and the resulting mixture was spun down at 13,000 rpm for 5 minutes. Finally, 200 μL of the supernatant was diluted into 1.0 mL of the initial mobile phase.
Calibration standards were injected in duplicate, 6 separate quality control (QC) samples were prepared at each of the QC concentration points. The QC samples were injected once each.
|
LC system: |
ACQUITY UPLC System |
|
Column: |
ACQUITY BEH C18 2.1 x 50 mm, 1.7 μm |
|
Eluent: |
85:15 water/acetonitrile (isocratic) |
|
Run time: |
0.6 min |
|
Injection volume: |
5 μL |
|
MS system: |
Quattro Premier Tandem Quadrupole Mass Spectrometer |
|
Ion mode: |
Electrospray positive |
|
Capillary voltage: |
3.00 kV |
|
Cone voltage: |
25 V |
|
Detection mode: |
MRM (195.1>137.9 Da) |
|
Collision energy: |
20 V |
|
Dwell time: |
0.1 sec |
|
Collision gas: |
Argon (3.4x10-3 mbar) |
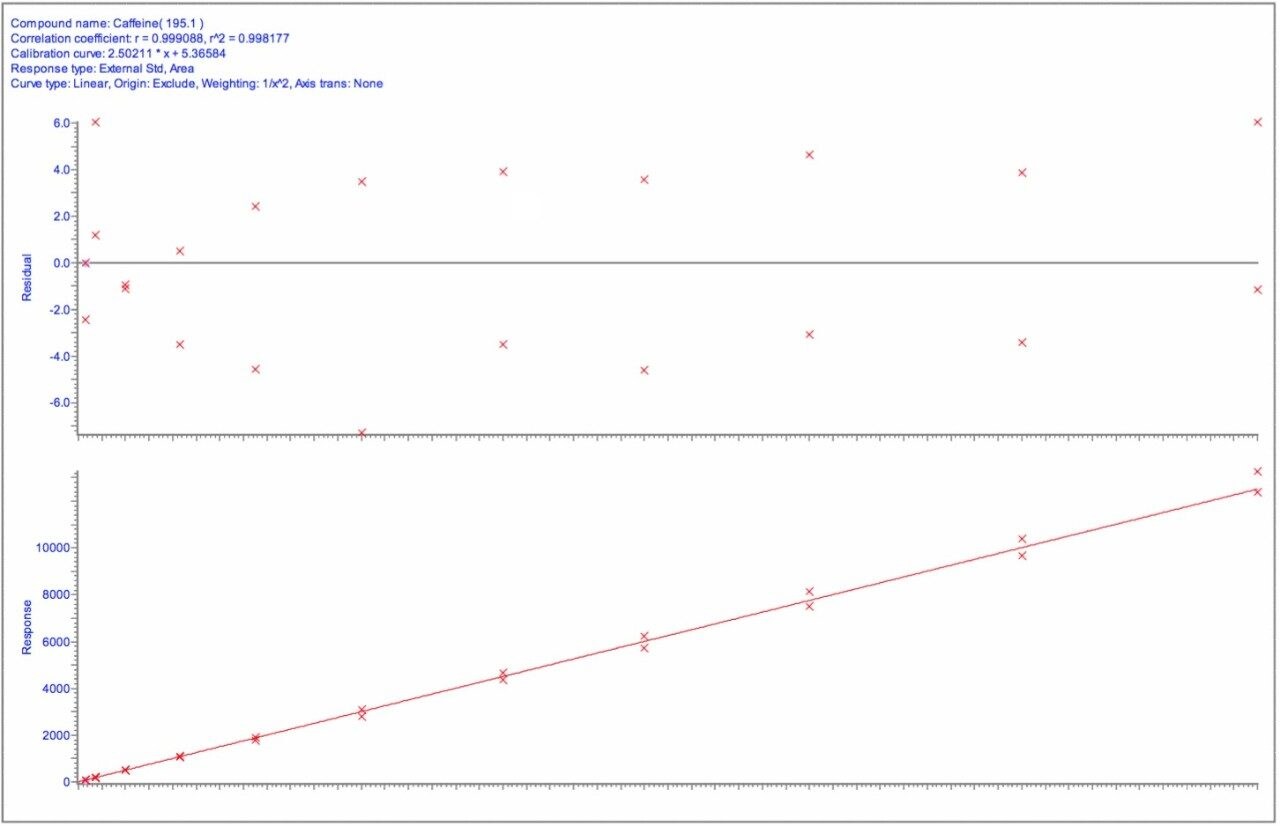
A full validation batch was run on each of 3 separate days. A typical calibration curve is shown in Figure 1. The calibration curve was plotted using a linear fit with a 1/x2 weighting, and gave a coefficient of correlation of >0.998.
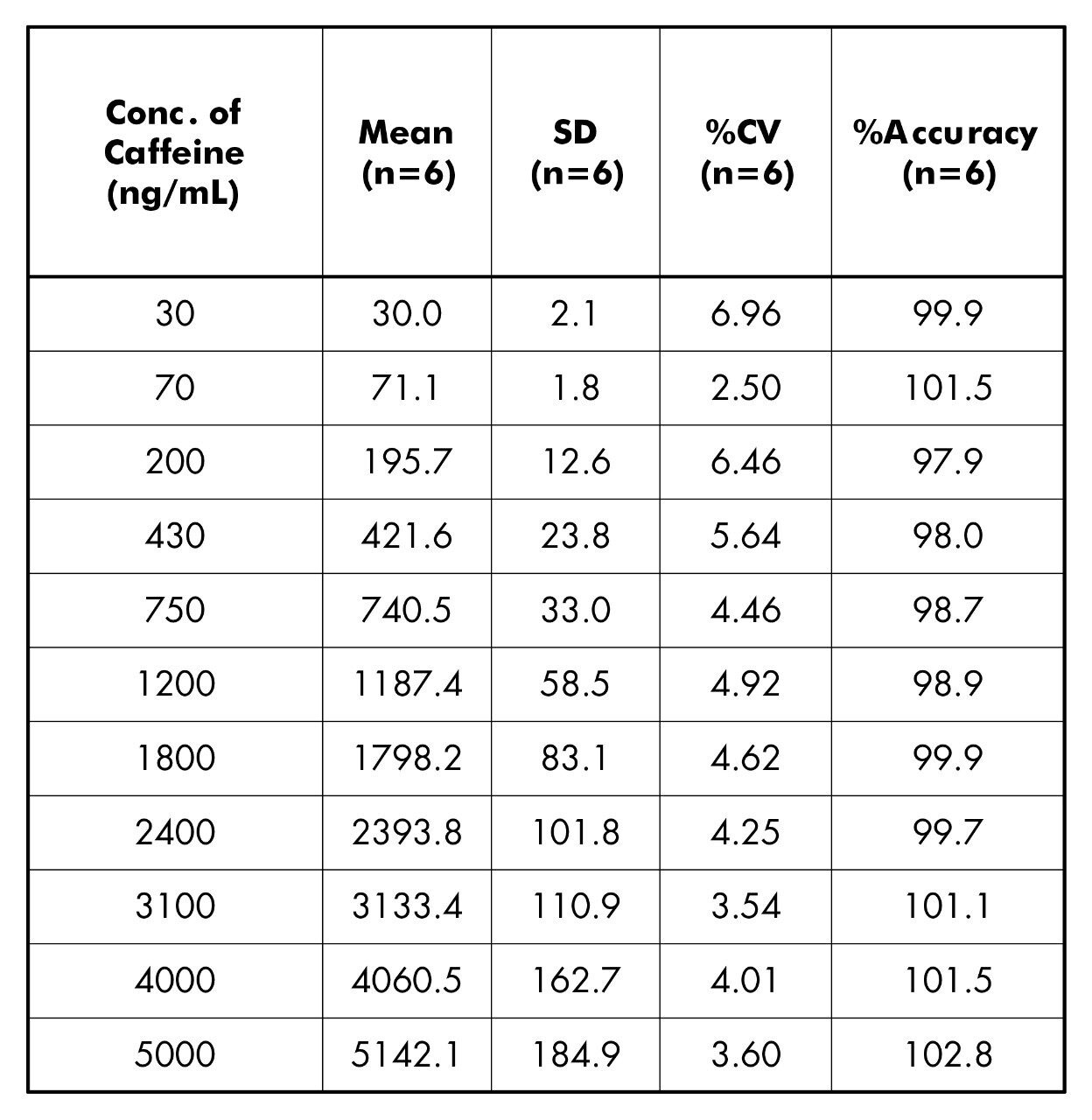
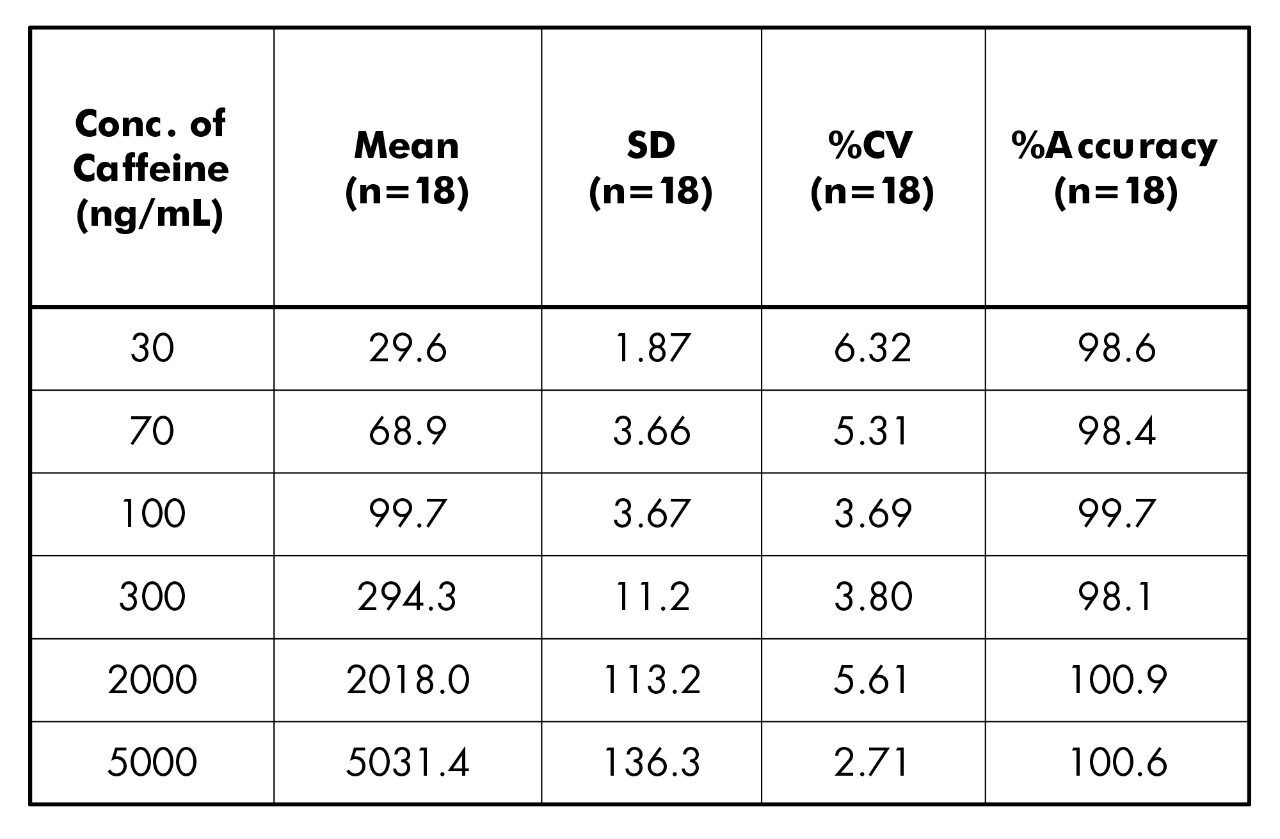
Shown in Tables 2 and 3 are the inter-batch statistics for the standard and QC samples.The statistics for the standard injections are based on 2 replicate injections of the 11 calibration points for each of the 3 inter-day batches. All calibration points show <7% CV with accuracy valuesbetween 97.9%–102.8% (Table 2). The statistics for the QC injections are based on single injections of 6 individually spiked QC solutions at each concentration for each of the 3 inter-day batches. All QC levels show <6.4% CV with accuracy values between 98.1% –100.9% (Table 3).
The ACQUITY UPLC System with the Quattro Premier Tandem Quadrupole Mass Spectrometer have been combined to yield an unmatched platform for quantitative LC-MS/MS. The results demonstrate the excellent accuracy and precision that can be achieved for compounds in complex biological matrices, such as human plasma. In this case, the required criteria were easily met without the use of an internal standard. With the enhanced chromatographic capabilities of UPLC, this analysis wasachieved with a run time of 0.6 minutes. This shows that accuracy and precision can be achieved with very fastrun times, giving the potential to significantly increase throughput as compared to an HPLC-based system, yet still maintain the necessary selectivity and resolution for good quality data. The target LLOQ of 30 ng/mL is much higher than could potentially be achieved with UPLC-MS/MS, but was determined to be sufficient for the dosage levels to be detected.
720001413, November 2005