Improving Sample Throughput of HPLC Methods Using CORTECS 5 µm Columns
Kenneth Berthelette, Maureen DeLoffi, Thomas H Walter
Waters Corporation, United States
Published on October 28, 2025
Abstract
Validated HPLC methods are prime targets for systematic improvements like increasing throughput or reducing solvent usage. One way to attain these benefits is to use a column packed with solid-core particles. This application brief examines improving the throughput and analytical greenness of a validated HPLC method by migrating from a column packed with 5 µm fully porous particles to a CORTECS™ Premier C18 5 µm Column. Chromatographic performance, system pressure, and Analytical Method Greenness Scores (AMGS) are compared across the columns tested.
Benefits
- Comparable chromatographic performance for abacavir impurities was achieved using a shorter CORTECS Premier C18 5 µm Column in place of a column packed with fully porous 5 µm particles
- 33% reduction in solvent usage and analysis time using a CORTECS Premier C18 5 µm Column compared to a fully porous 5 µm column
- 33% reduction in analytical method greenness score indicating improved sustainability for the new method
Introduction
Improving validated HPLC methods can be challenging. Two of the approaches to increase analytical throughput, increasing mobile phase flow rate or using shorter columns, have drawbacks in higher pressures or reduced chromatographic performance. Typically, using a shorter column also requires decreasing the particle size to maintain a constant length to particle size (L/dp) ratio to ensure similar column efficiency. However, columns packed with smaller particles generate higher back pressures. Some labs may be able to migrate methods to shorter columns packed with smaller particles by transferring to a HPLC system capable of operating at higher pressures. However, if a laboratory lacks access to such systems, then their options may be limited. In those situations, finding appropriate columns to use to improve a method is not only a matter of chromatographic performance but also system compatibility, particularly from a pressure standpoint. Older HPLC systems are limited to lower pressures compared to more modern systems.
This application brief examines the improvement of a method for testing abacavir impurities by migrating the original method from a 4.6 x 150 mm 5 µm fully porous C18 column to a CORTECS Premier C18 4.6 x 100 mm 5 µm Column. Additionally, a fully porous 3.5 µm C18 column in 4.6 x 100 mm hardware was evaluated to determine if moving to a smaller particle size would have as much of a benefit as that provided by using a CORTECS Column. The separation conditions for method migration were obtained using the Waters Column Calculator. Along with examining throughput, solvent savings were calculated as well as analytical method greenness scores for each column tested.
Experimental
Sample Description
The abacavir impurities mixture was purchased from Sigma-Aldrich and diluted to 1 mg/mL with water.
Method Conditions
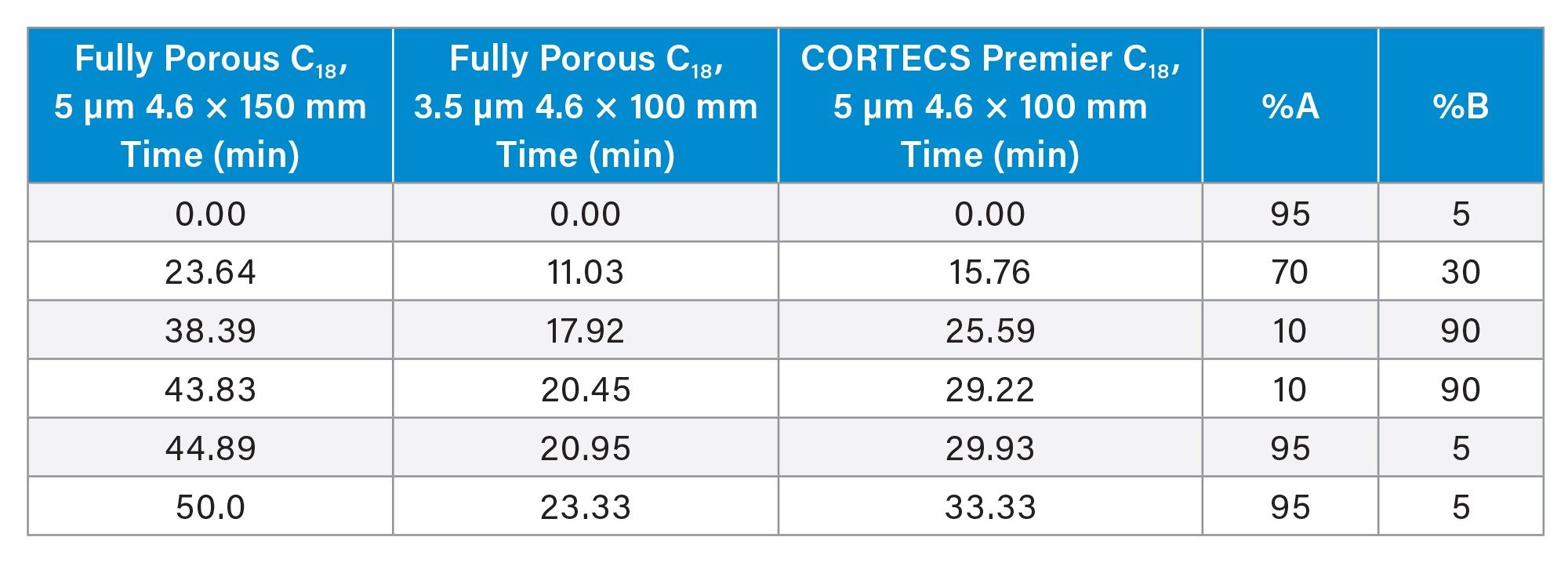
|
LC system: |
Alliance™ HPLC System with 2489 UV/Visible (UV/Vis) Detector |
|
Detection: |
UV @ 254 nm |
|
Columns: |
CORTECS Premier C18 Column, 4.6 x 100 mm, 5 µm (p/n:186010791) Fully Porous Silica C18, 4.6 x 150 mm, 5 µm Fully Porous Silica C18, 4.6 x 100 mm, 3.5 µm |
|
Column temperature: |
30 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
8.0 µL (150 mm column), 5.3 µL (100 mm columns) |
|
Flow rate: |
1.0 mL/min (5 µm columns), 1.43 mL/min (3.5 µm column) |
|
Mobile phase A: |
0.1% TFA in Water |
|
Mobile phase B: |
Methanol:Water (85:15 v/v) |
|
Gradient conditions: |
See Table 1. |
Data Management
|
Chromatography software: |
Empower™ Chromatography Data System (CDS) |
Gradient Table
Results and Discussion
Previously, the impurity method for abacavir was migrated to a 2.7 µm CORTECS Column.1 While that work showed the benefits of scaling to a smaller particle size, not all laboratories are comfortable moving to such a small particle size. In those instances, moving from a 5 µm to a 3.5 µm particle column is a good first step. However, depending on the method, this migration might not be appropriate and could result not only in elevated system pressure but also performance loss. Figure 1 shows the chromatograms for abacavir impurities using the original method column, a 3.5 µm C18 column, and the CORTECS Premier C18 5 µm Column.
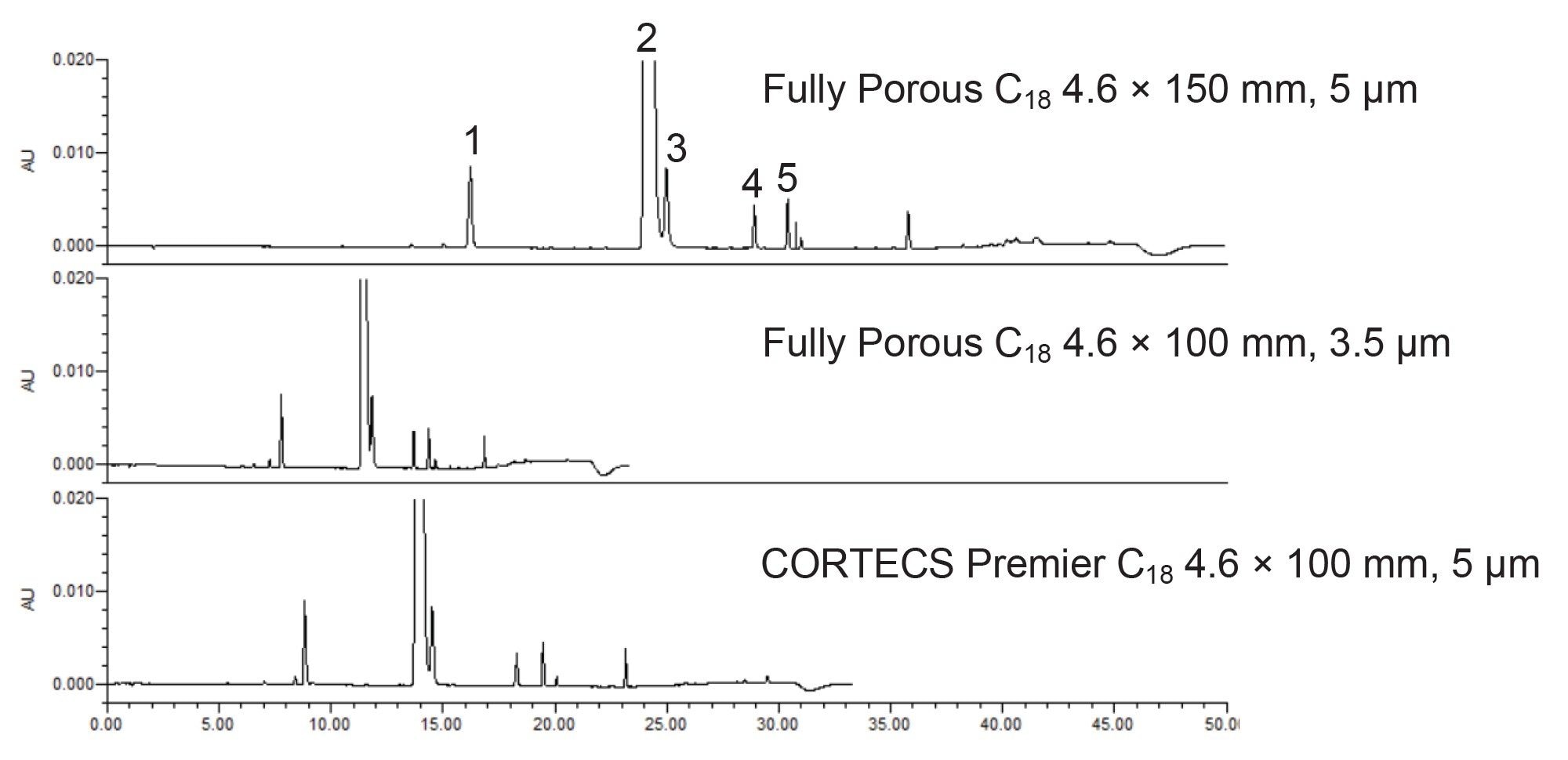
As expected, the use of the shorter column lengths, regardless of particle size employed, leads to shorter analysis times. The fully porous 3.5 µm particle column has a run time of 23.3 minutes compared to the original 50.0 minute run time. While this improvement is certainly beneficial, the overall system pressure and chromatographic performance must be examined to determine if the separation is equivalent to the original. Table 2 shows the average (n=3) USP resolution for peaks 2 and 3 across the three columns tested as well as the total system pressure. System pressure was recorded at the apex of pressure for the gradient run.
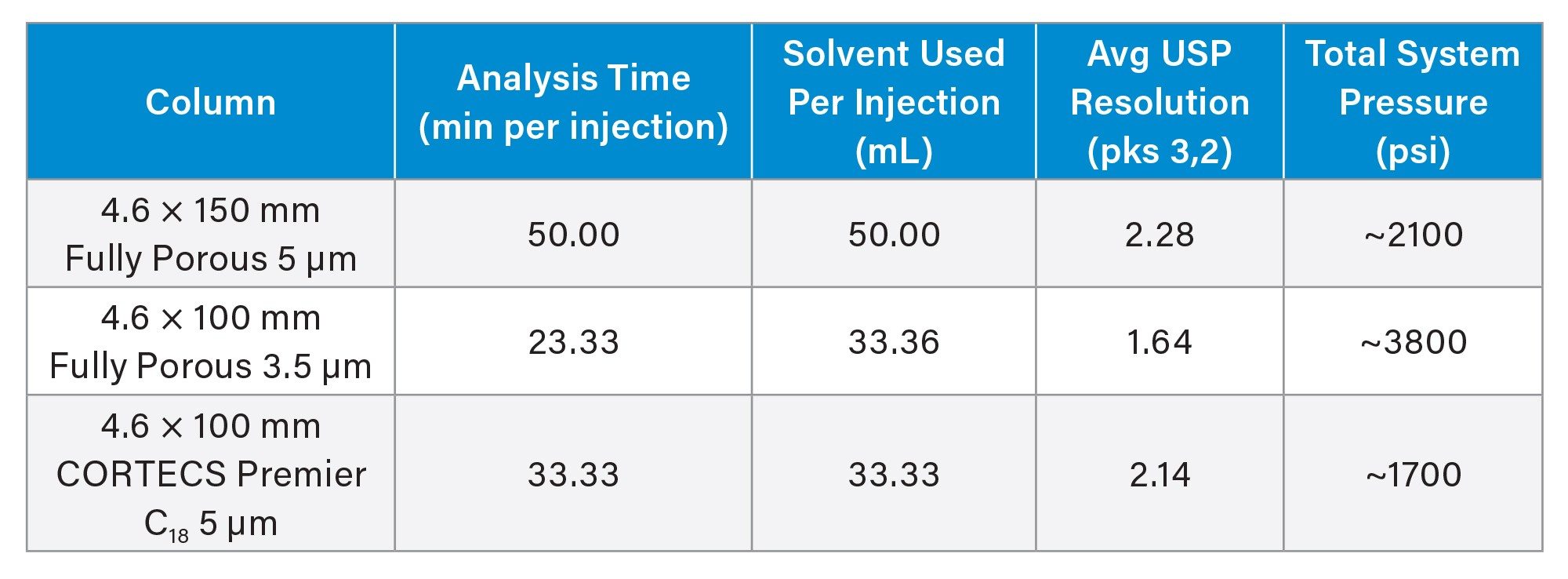
Using the 3.5 µm particle column resulted in slightly lower chromatographic performance, as measured by USP resolution for the critical pair. Additionally, the system experienced pressure near the 5,000 psi operating limit. For this sample, the use of the 3.5 µm particle column may have improved throughput and solvent usage, but at the cost of chromatographic performance. It should be noted that even with this loss of resolution the peaks are still considered baseline resolved as the USP resolution value is >1.5.
Employing a CORTECS Premier C18 5 µm Column for this separation, however, yields comparable chromatographic performance and system pressure relative to the original conditions, while also achieving higher throughput. This is possible due to the solid-core particles that are used in these columns. The benefits of solid-core particles compared to fully porous particles of the same approximate size are well documented.2-4 From a practical standpoint, the increased efficiency gained with CORTECS Columns allows a shorter 100 mm column to be used to achieve approximately the same efficiency as a 150 mm column packed with fully porous 5 µm particles.
In addition to seeing an improvement in throughput and reduced solvent usage, the AMGS for this separation is greatly improved using the CORTECS Premier C18 5 µm Column. The AMGS metric was created by a group of scientists from several pharmaceutical companies to compare the environmental impacts of different separation methods so that more sustainable methods could be selected.5 Previous work has shown the benefits of using AMGS values for comparing methods.6-8 Table 3 shows the AMGS for the separation of abacavir impurities on each column tested using the method parameters outlined previously. Lower AMGS values indicate a more sustainable method.
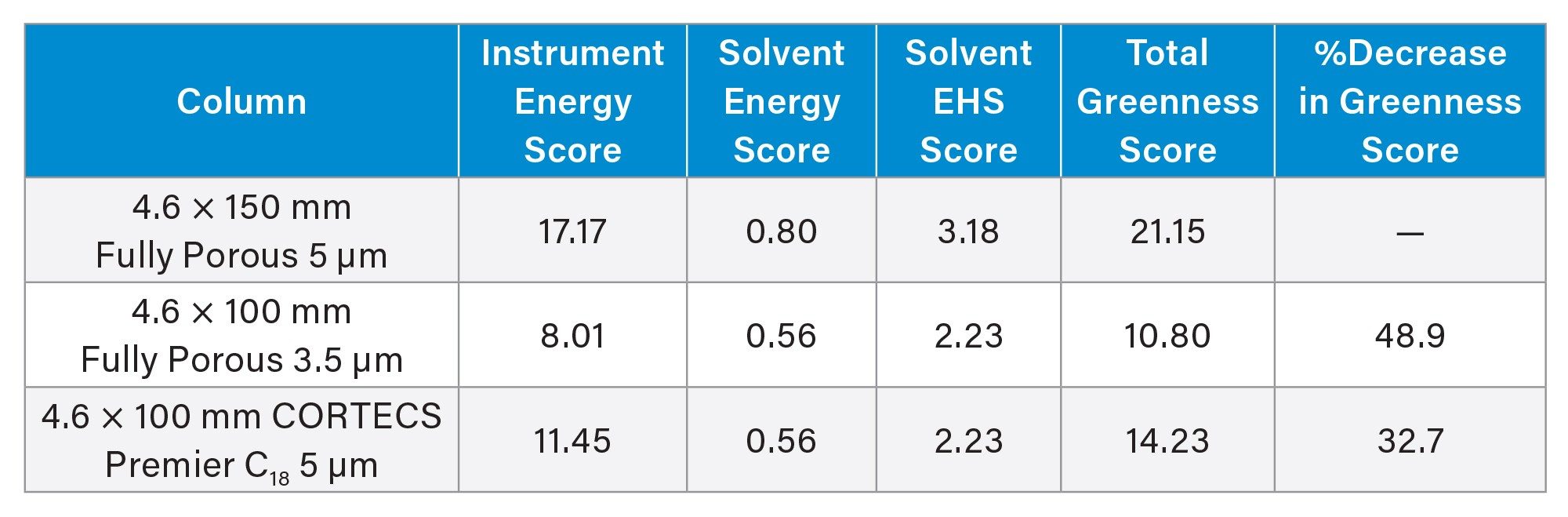
The CORTECS Premier C18 Column method has a 32.7% lower total greenness score compared to the original method. It should be noted that the most sustainable method, i.e. lowest AMGS, used the fully porous 3.5 µm particle column. This is due to the lower instrument energy score, which factors in analysis time and the energy consumption of the instrument for the duration of that analysis. Since the method using the fully porous 3.5 µm particle column had a shorter run time, the energy needed to run the instrument is less, leading to a better total greenness score. However, a lower AMGS value does not compensate for the chromatographic performance loss seen for this method.
Using the CORTECS Premier C18 5 µm, 100 mm Column not only provided comparable separation performance as a 150 mm column packed with fully porous 5 µm particles, it did so with a shorter run time, less solvent used, lower system pressure, and a more sustainable overall method, as measured by the AMGS metric. Utilizing these columns can improve validated methods without having to change the HPLC system.
Conclusion
CORTECS Premier 5 µm Columns are suitable alternatives for fully porous 5 µm columns for improving validated HPLC methods. Using a CORTECS solid-core stationary phase can allow for a shorter column to be used because of their higher efficiency per unit length. To highlight this, the impurity method for abacavir was migrated from the original fully porous 5 µm, 4.6 x 150 mm column to a CORTECS Premier C18 5 µm, 4.6 x 100 mm Column, as well as a 4.6 x 100 mm 3.5 µm fully porous column. The use of the CORTECS Column achieved comparable separation performance as the original while also exhibiting a shorter analysis time, lower solvent usage, and a better AMGS value.
References
- Turner J, Alden B, Summers M, Berthelette K, Fountain K. Transferring HPLC Gradient Methods Using CORTECS Solid-Core Particle Columns. Waters Application Note. 720005104.
- Broeckhoven K, Cabooter D, Desmet G. Kinetic performance comparison of fully porous and superficially porous particles with sizes ranging between 2.7 µm and 5 µm: Intrinsic evaluation and application to a pharmaceutical test compound. J. Pharm Anal. (2013) 3(5):313–323.
- Fekete S, Guillarme D. Superficially Porous Particles: Perspectives, Practices and Trends. LCGC Europe. (2014) 27;6 312–323.
- Berthelette K, Summers M, Fountain K. Improving Resolution using CORTECS UPLC Columns. Waters Application Note. 720004737.
- Hicks MB et al. Making the move towards modernized greener separations: introduction of the analytical method greenness score (AMGS) calculator, Green Chem. (2019) 21, 1816–1826.
- Berthelette K, Water TH, Collins C, DeLoffi M, Haynes K. Applying Analytical Method Greenness Scoring to the USP Monograph of Naproxen; Improving Sustainability of Validated Methods by Modernizing to Newer Technology. Waters Application Note. 720008366.
- Berthelette K, Walter TH, DeLoffi M, Kalwood J, Haynes K. Creating Greener HPLC Methods as Measured by the AMGS Metric: A Case Study of Improving USP Monograph Methods. Waters Application Note. 720008281.
- Dlugasch A, Hong P. Taking Advantage of 12k psi Pressure Capabilities for Modernizing USP Methods on the Alliance iS HPLC System. Waters Application Note. 720008973.
720009072, October 2025