Application Brief
This is an Application Brief and does not contain a detailed Experimental section.
Samantha Ippoliti, Brad Williams, Dale Cooper-Shepherd
Waters Corporation, United States
Published on January 06, 2026
This is an Application Brief and does not contain a detailed Experimental section.
Aspartic acid (Asp) isomerization in therapeutic proteins can impact safety and efficacy, making accurate characterization essential. Conventional peptide mapping often fails to fully resolve isomeric species such as L-Asp, D-Asp, L-isoAsp, and D-isoAsp due to their subtle structural differences and identical elemental composition. In this study, the combined power of high-resolution cyclic ion mobility spectrometry (IMS) and electron capture dissociation (ECD) for deep characterization of Asp and isoAsp variants in monoclonal antibody peptides is demonstrated. Using the SELECT SERIES™ Cyclic IMS Mass Spectrometer with an integrated ECD cell, complete separation of all four isomeric forms in a synthetic peptide mixture and in spiked digest samples was achieved, where chromatographic resolution alone was insufficient. Furthermore, ECD fragmentation provided definitive identification of isoAsp residues through characteristic product ions, enabling confident differentiation between Asp and isoAsp species. This workflow offers unprecedented clarity in the analysis of aspartic acid isomerism, supporting robust biotherapeutic characterization.
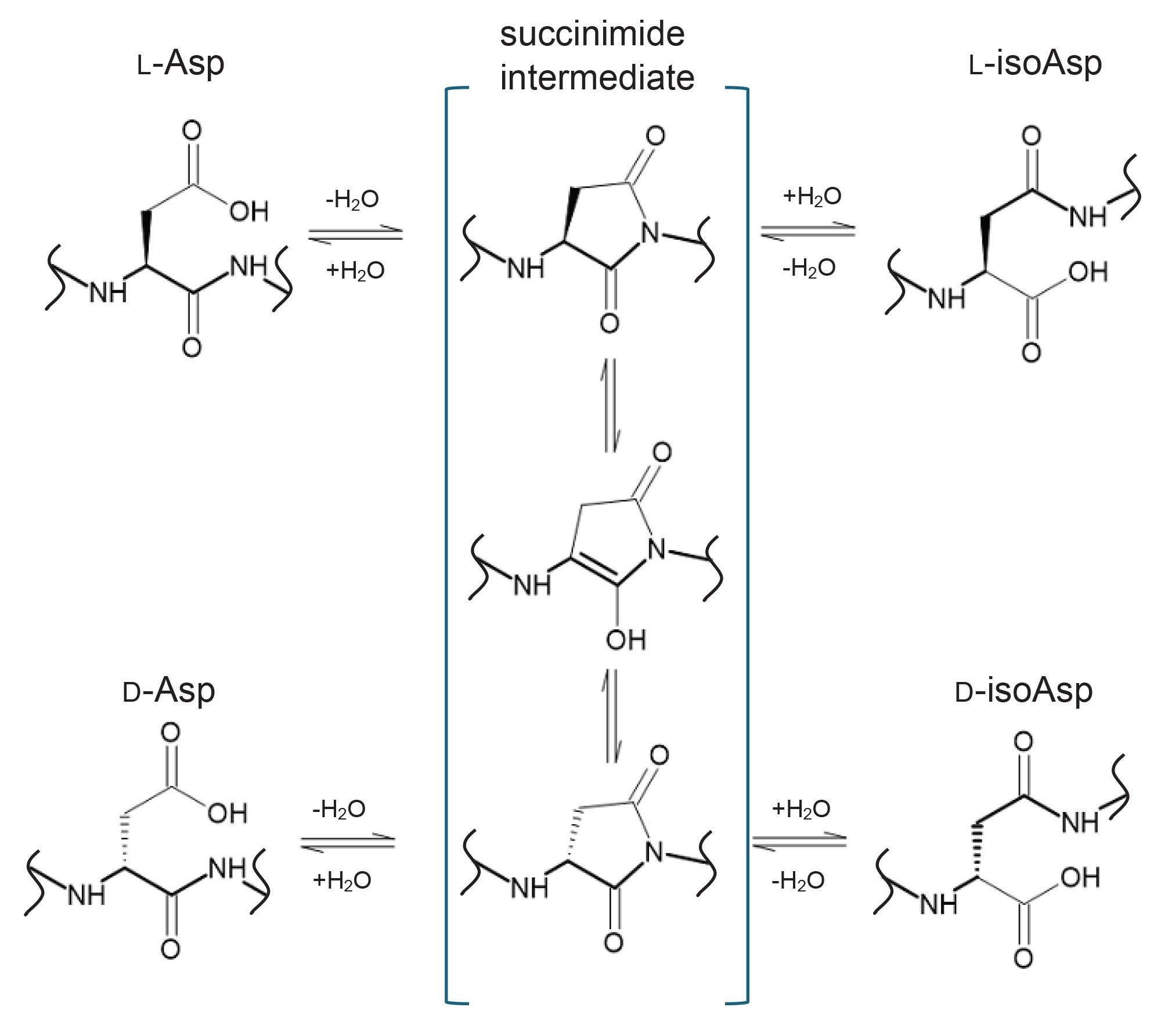
Isomeric amino acid residues have an effect on protein function, sometimes significant enough to contribute to diseases such as Alzheimer’s disease.1 In the realm of biopharmaceutical drug products, these isomerized protein species can have undesired effects on safety or efficacy and therefore must be investigated and characterized. The introduction of structural isomers and stereoisomers can occur during cell culture, downstream processing, or while in storage.1,2 Asp, specifically, can be present in a protein in four forms: L-Asp, D-Asp, L-isoAsp, or D-isoAsp. The most common form is L-Asp, which can isomerize or racemize into the other three forms through a succinimide intermediate (Figure 1).1 This modification is particularly challenging to characterize as it is a relatively small spatial change that is isomeric and therefore cannot be distinguished by typical characterization methods such as mass spectrometry. Various methods for the investigation of Asp and isoAsp are being employed in the industry today. The most common is peptide mapping, in which the protein is enzymatically digested and the resulting peptides are separated chromatographically in order to quantify isomer species by either UV or ion peak areas.3
Cyclic ion mobility mass spectrometry is highly powerful in the separation of isomeric species, including small molecules,4 natural products,5 lipids,6 peptides,7 and oligonucleotides.8 By separating on the basis of molecular shape in a high-resolution ion mobility device, Cyclic IMS provides a means to distinguish ions with identical elemental formulae but distinct arrangements of atoms in space.
Collision-induced dissociation is by far the most widely used fragmentation technique in tandem MS studies, providing high efficiency fragmentation with broad applicability in the structural elucidation of both small and large molecules. However, some types of isomerism are out of reach of CID, which leads to the need for alternative techniques. ECD is an emerging tool capable of identifying peptide isomers directly from tandem MS spectra, depending on the type of isomerism.9,10 It is well established that ECD can distinguish some isomeric amino acids in leucine- and isoleucine-containing peptides, as well as Asp- and isoAsp-containing peptides, by virtue of characteristic product ions. In the latter case, for an isomerization at position n in a peptide of length m, characteristic ions c(n-1)+57 Da and z(m-n-1)-57 Da can be observed. For further information on comprehensive biotherapeutic characterization using ECD, there is an existing Waters application note.11
In this application brief, the depth of characterization achieved using the SELECT SERIES Cyclic IMS Mass Spectrometer equipped with the Waters ECD cell option is demonstrated. The power of high-resolution cyclic ion mobility enables full separation of the four isomer mix of a mAb tryptic peptide with L-Asp, L-isoAsp, D-Asp and D-isoAsp forms, where only partial chromatographic separation is achieved. Furthermore, ECD fragmentation clearly distinguishes the Asp isomers from their isoAsp counterparts, providing unprecedented clarity in the analysis.
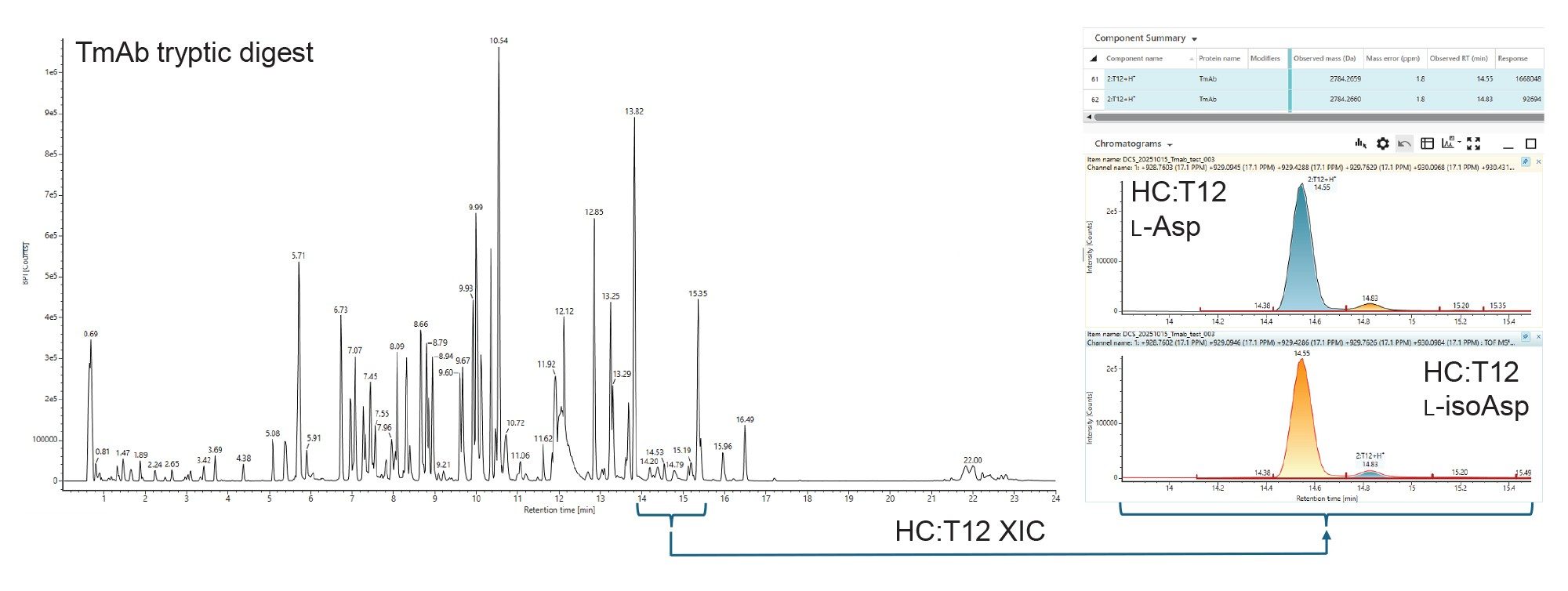
During routine peptide mapping of the mAb Trastuzumab, two major isomeric peaks for the T12 peptide from the heavy chain were detected. In cases such as this, the major peak is the native peptide, and the minor peak is putatively identified as the isoAsp variant (Figure 2). These two variants are well separated in retention time, leading to facile relative quantification.
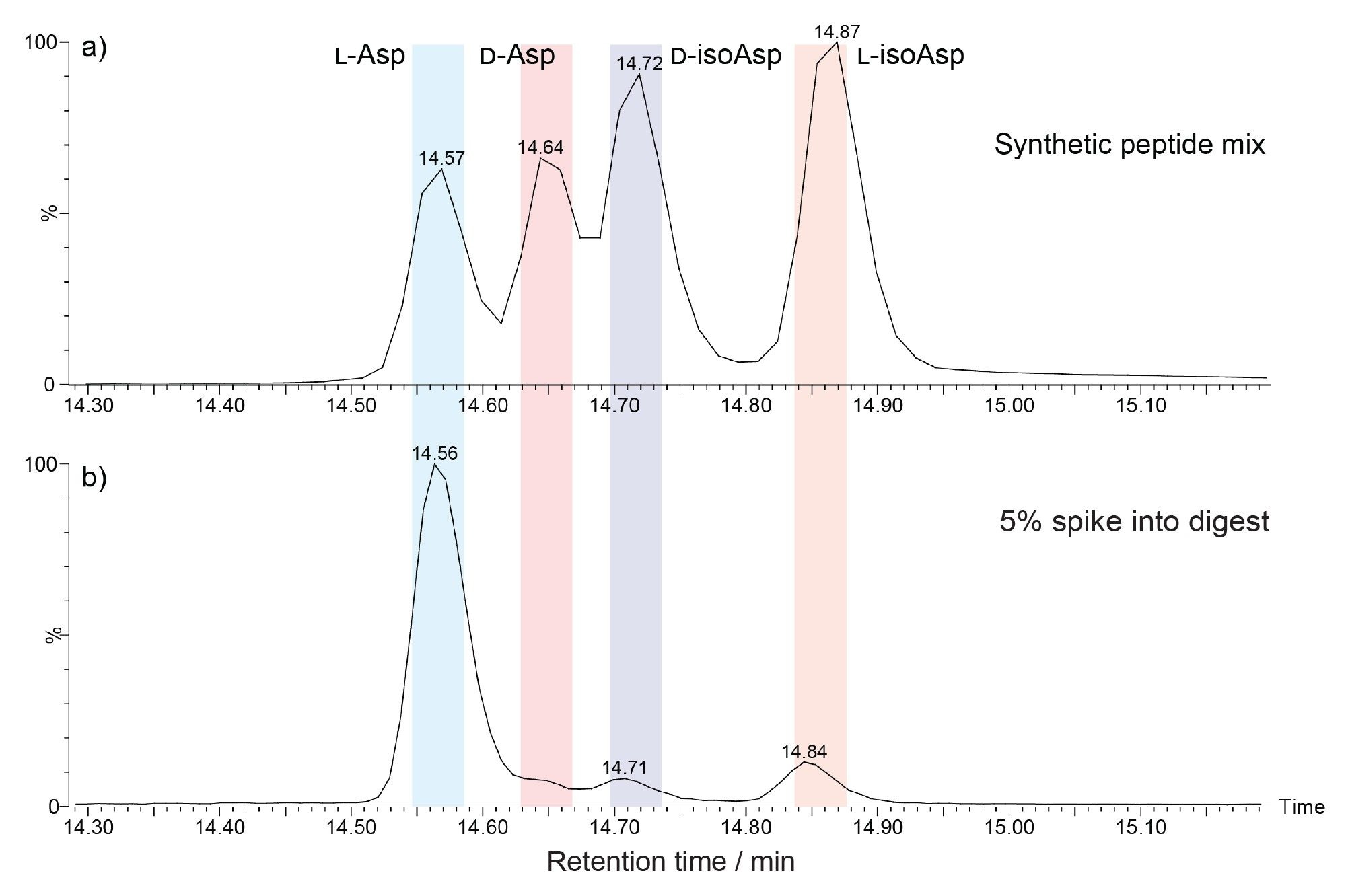
Given the potential of the native L-Asp-containing peptides to racemize to the D-Asp form, the ability of the peptide mapping assay to fully separate a mixture of peptides populating the L-Asp, L-isoAsp, D-Asp and D-isoAsp variants was assessed. For this, synthetic forms of the HC:T12 peptide with sequence WGGDGFYAMDYWGQGTLVTVSSASTK were purchased, with all Asp isomeric residues at position 4. Performing LC-MS of the mixture of the four synthetic HC:T12 peptides yielded a chromatogram with four peaks, three of which are partially resolved (Figure 3a). Resolution of co-eluting components is made more challenging still when there are minor components in the mix. This can be seen in the chromatogram in Figure 3b, where the synthetic peptides were each spiked at a 5% level into the trastuzumab tryptic digest sample. This chromatogram shows that the species at 14.6 and 14.7 minutes are considerably less simple to distinguish from the L-Asp peak at 14.56 minutes.
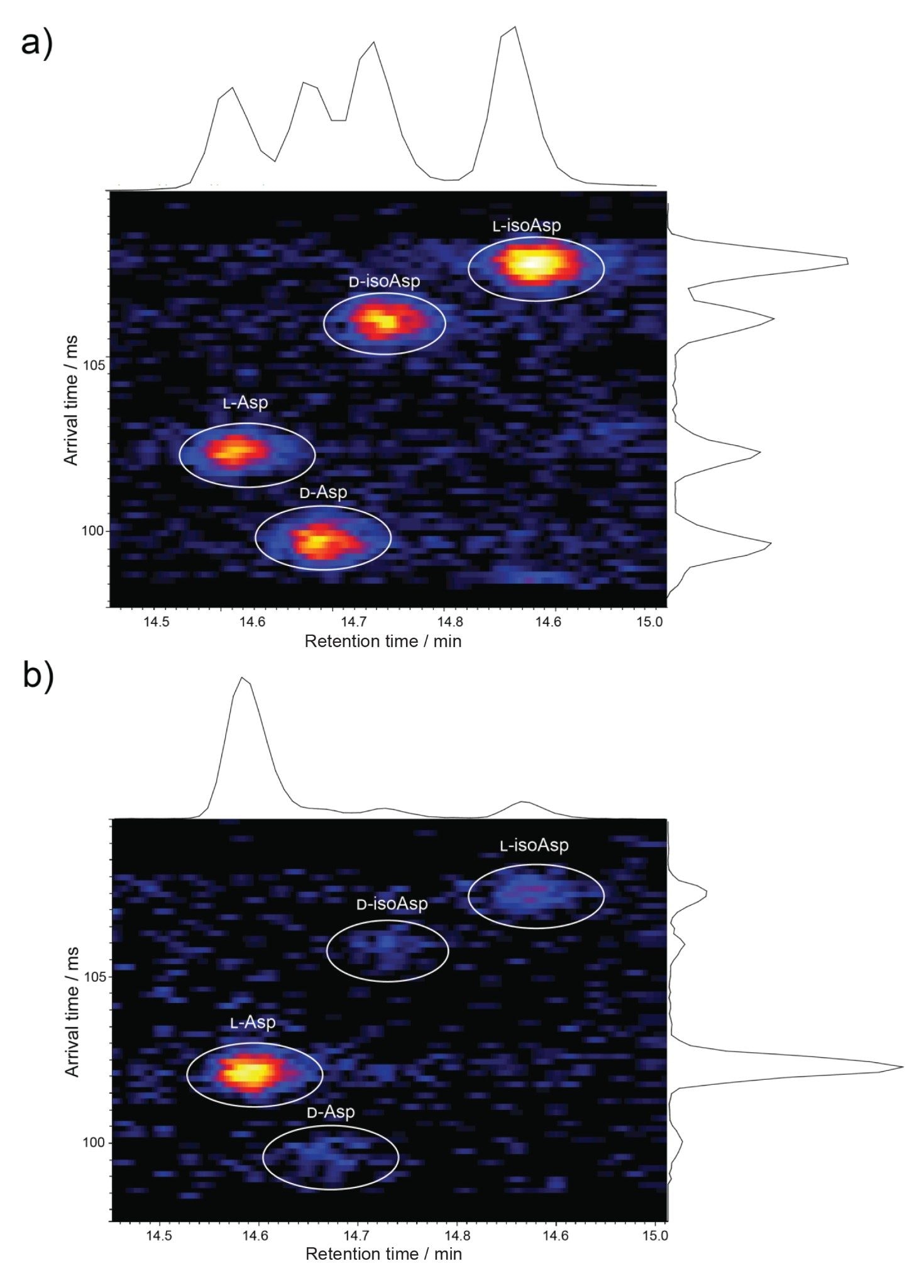
To address this analytical challenge, these two samples were subjected to multipass cyclic ion mobility (10 passes in total). Figure 4 shows the resulting LC-Cyclic IMS-MS data for the 4+ charge state of the HC:T12 peptides at 696.85 m/z. For the synthetic peptide mixture (Figure 4a), complete resolution of the four species is observed at the arrival time versus retention time heat map, revealing unprecedented clarity in the separation. Furthermore, even in the spiked sample (Figure 4b), all four species are clearly separated. This high-resolution cyclic ion mobility separation in combination with LC-MS provides a means to confidently identify and accurately quantify these isomeric forms in biotherapeutic digests.
Taking the work a step further, ECD fragmentation to differentiate the Asp and isoAsp forms in the mixture was used. Employing direct infusion electrospray ionization of the 4-way isomer mixture, 10 pass cyclic IMS separation was performed on the 4+ charge state, followed by ECD fragmentation. For these experiments, the ECD cell was positioned in the post-IMS configuration, meaning that ECD product ions are encoded with the arrival time of their corresponding precursor ions (Figure 5). For the HC:T12 peptide, the characteristic ions indicative of the presence of isoAsp are c3+57 and z23-57, with m/z values of 375.16 and 1206.06, respectively. Extracted ion mobilograms of these ions (Figure 5a, blue and red) revealed arrival times consistent with the two lower mobility species, confirming these to be L-isoAsp and D-isoAsp in agreement with the data above.
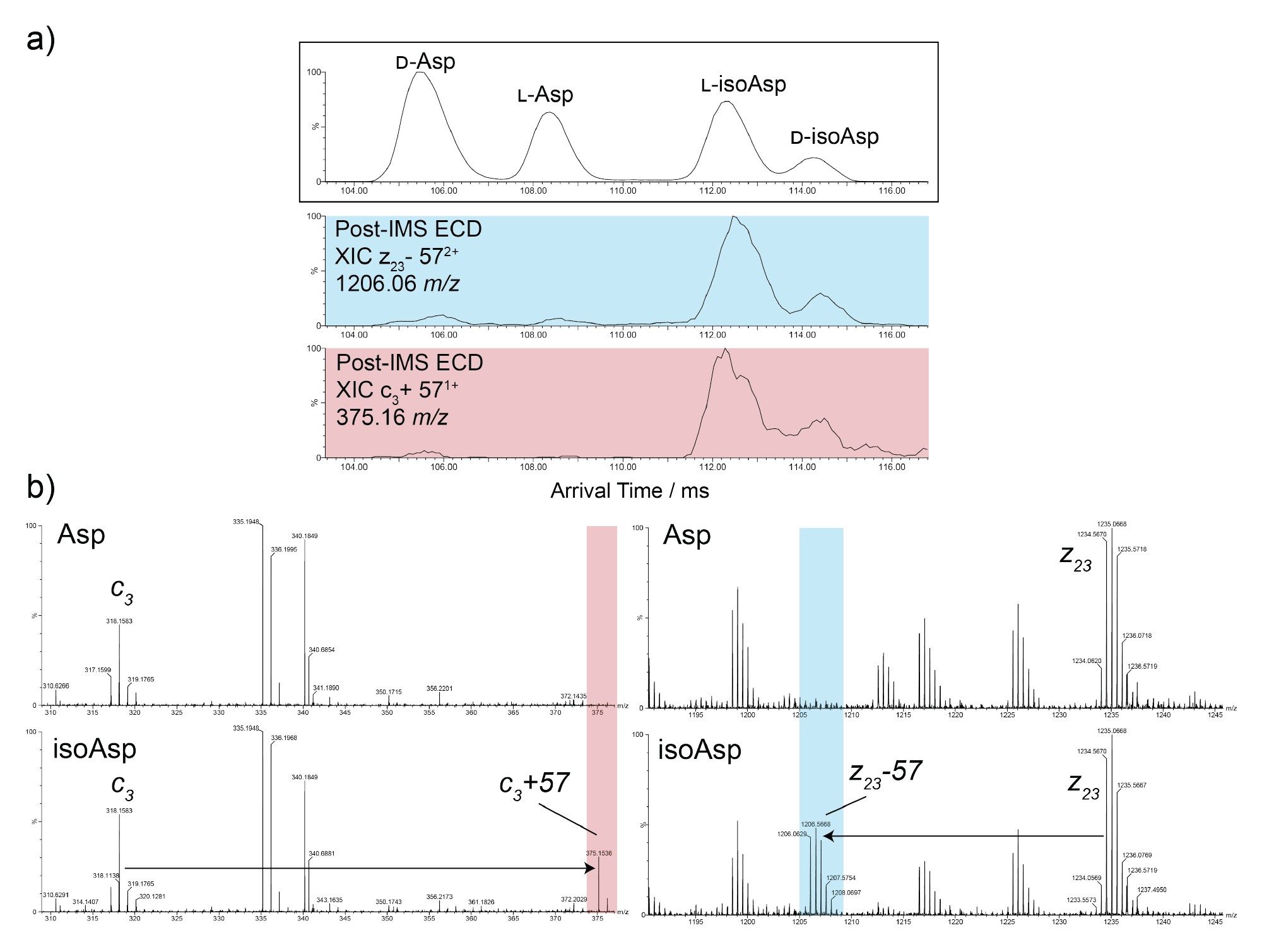
High-resolution cyclic IMS combined with ECD fragmentation delivers a powerful solution for resolving and identifying aspartic acid isomers in complex biotherapeutic samples. Unlike conventional LC-MS peptide mapping, which provides only partial separation, multipass cyclic IMS achieves complete resolution of L-Asp, D-Asp, L-isoAsp, and D-isoAsp forms, even at low abundance. The integration of ECD further enhances specificity by generating diagnostic fragment ions that distinguish Asp from isoAsp residues. This approach reduces reliance on synthetic standards, accelerates analysis, and improves confidence in structural characterization. Overall, the workflow represents a significant advancement for monitoring critical quality attributes in monoclonal antibodies and other protein therapeutics.
720009185, December 2025