Comprehensive Workflow for the Quantification of Peptides and Proteins in Plasma: Semaglutide– a Case Study
Samantha Ferries
Waters Corporation, United States
Published on November 11, 2025
Abstract
Over the last two decades, there has been a significant increase in protein and peptide-based therapeutics. “Biologics” accounted for 21 of the 55 new medicines approved in 2023, most notably the glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide. These protein and peptide therapies typically show high potency and specificity. They also have a long pharmacokinetic (PK) half-life, resulting in nmol/L or lower concentration levels in the circulatory system, which requires a high sensitivity bioanalytical assay to accurately define the compound PK. The development of a suitable LC-MS/MS method for the quantification of proteins and peptides in biofluids is complicated by the formation of precursor ions with multiple charge states and the large number of possible product ions to be evaluated. Here, the use of an automated workflow is demonstrated to select, optimize, and compare peptide multiple reaction monitoring (MRM) transitions. This workflow uses waters_connect™ for Quantitication Software and the MRM Optimization tool for the high sensitivity quantification of semaglutide in human plasma using the Xevo™ TQ Absolute Mass Spectrometer coupled with an ACQUITY™ Premier UPLC™ System.
Benefits
- Xevo TQ Absolute Mass Spectrometer enables sensitivity quantification of semaglutide at sub ng/mL levels (LLOQ of 0.2 ng/mL) enabling accurate determination of PK elimination phase.
- Oasis™ MAX µElution mixed-mode SPE Plates deliver high selectivity for isolation of semaglutide from human plasma, enabling analyte concentration, cleaner extracts, and improved sample recovery.
- MaxPeak™ High Performance Surfaces (HPS) Technology: Mitigates non-specific binding facilitating increased sensitivity through high peptide recovery and improved peak shapes with HPS in Waters QuanRecovery™ Plates, Premier Columns, and ACQUITY Premier UPLC System.
waters_connect for Quantitation Software Optimization tool was used to rapidly develop and evaluate the MRM transitions for quantification.
Introduction
The development of therapeutic peptides in drug discovery has significantly progressed over the past decade and now account for a significant proportion of the pharmaceutical market.1,2 Amongst the notable advancements in peptide therapeutics is the glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide, Figure 1. GLP-1 is an incretin hormone with metabolic effects including the stimulation of insulin secretion in response to glucose.3 GLP-1 agonists, are routinely used in the treatment of type 2 diabetes to ensure glycemic control and, more recently, for weight management/obesity.4 These GLP-1 receptor agonists are typically dosed once per week and exhibit long half-lives with circulatory concentrations in the low nmol/L range. Thus, to accurately define the pharmacokinetics (PK) of these compounds, it is critical to develop sensitive and robust bioanalytical assays to quantify these drugs and their metabolites at very low levels in blood extracts. Previous clinical studies3 have shown that 20 days after a 1 mg intravenous administration, the concentration of semaglutide had reduced to 0.5 nmol/L with systemic clearance of 0.0348 L/h. Therefore, to accurately determine the PK elimination phase of semaglutide, a high sensitivity, high specificity bioanalytical assay is required in the sub ng/mL range.
The sensitivity and specificity of LC-MS/MS is well suited to the quantification of low systemic concentration peptides like semaglutide. The development of bioanalytical assays for peptides, however, presents several challenges, namely in mass spectrometry (MS) method development. Key issues include eliminating non-specific binding and managing chromatographic peak shape, as peptides are well known to exhibit poor recovery and chromatographic peak shape due to non-specific binding to consumables and chromatographic components. The selection of the optimal MRM transitions for peptides is confounded by the numerous permutations of precursor and product ions, which is exacerbated by the fact that peptides form multiple charge state precursor ions. Evaluating all of these potential combinations of charge state/ precursor ion/ product ion/ collision energy is a daunting task, requiring the evaluation of multiple MRM transitions. Without tailored technologies and software, method development becomes a time-consuming task and presents risk to successful and efficient assay development.
To address these challenges of method development, optimization, and robust routine operation, Waters has developed a series of analytical tools to simplify peptide quantitative bioanalysis. The waters_connect Optimization tool has been designed to streamline the development of multiple reaction monitoring (MRM) methods for multiply charge biomolecules. MaxPeak ACQUITY UPLC Columns and Vials and ACQUITY Premier UPLC Chromatography System have been specifically designed with high performance surface technologies to mitigate and eliminate non-specific analyte binding to metal and glass surfaces. This application note describes a comprehensive workflow for the quantitative analysis of the GLP-1 peptide, semaglutide, by UPLC-MS/MS, using waters_connect for Quantitation Software, and ACQUITY Premier MaxPeak HPS Technologies to deliver lower limit of quantification (LLOQ) of less than 0.2 ng/mL in human plasma.

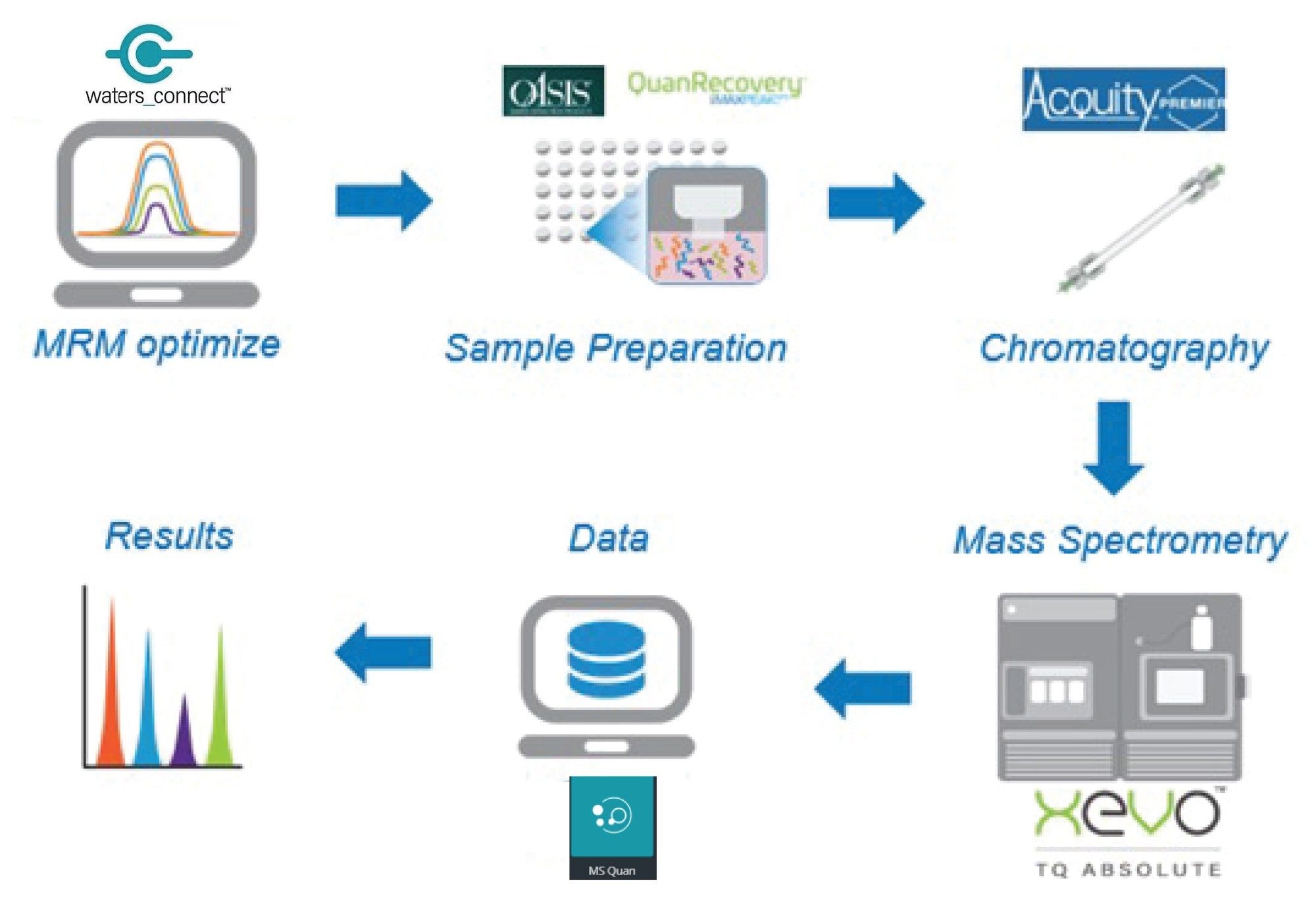
Experimental
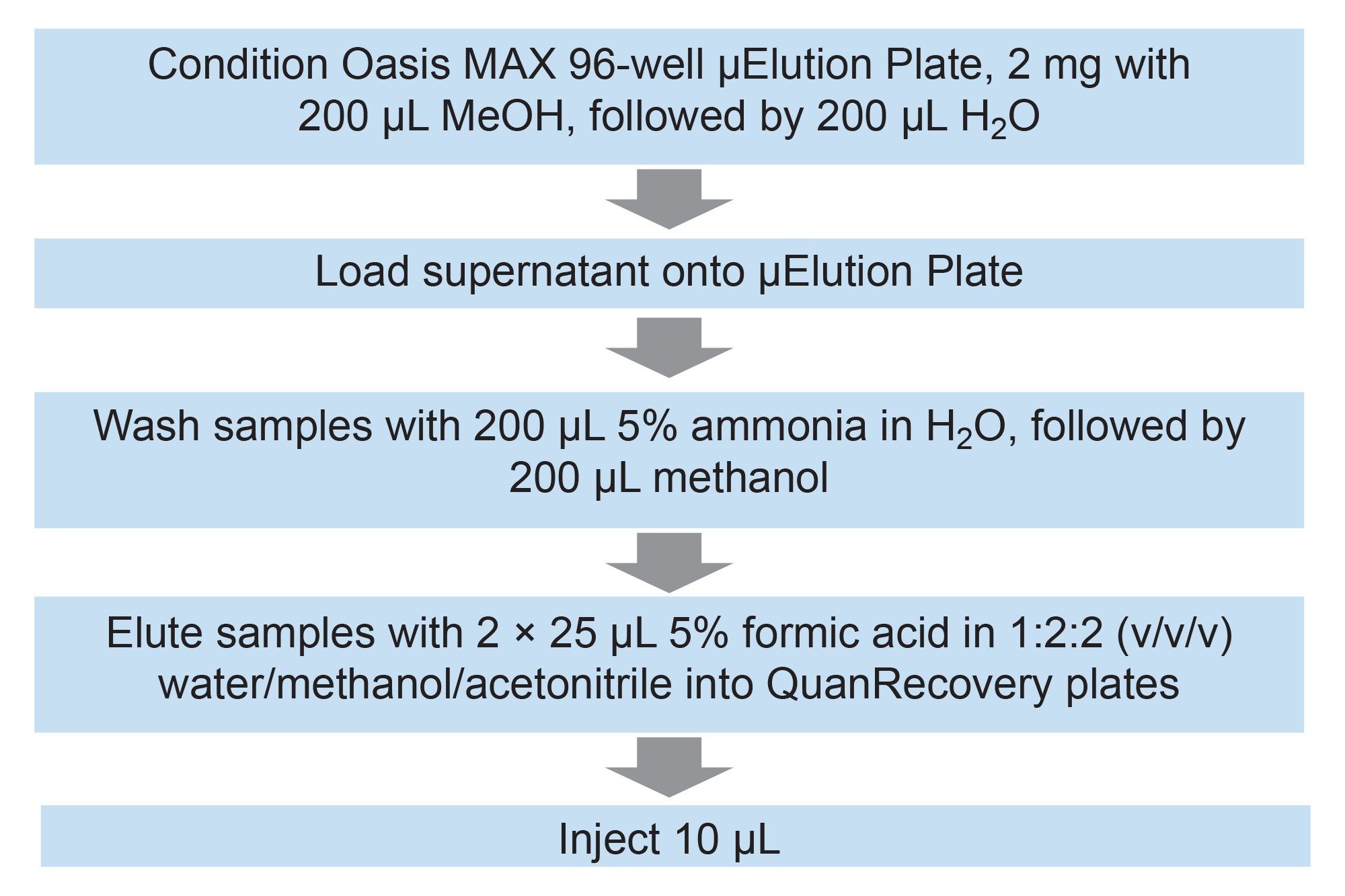
A calibration curve was prepared in human plasma by spiking with semaglutide (AstaTech Inc.) at 7 concentrations between 0.2 to 100 ng/mL. In addition, 5 replicate quality control (QC) standards were prepared in spiked human plasma at each of 4 concentrations (0.2, 0.3,7, and 80 ng/mL). Standards and QCs (200 mL) were extracted by addition of methanol (2:1 methanol: plasma) containing liraglutide as an internal standard (in the absence of a stable label isotope version of semaglutide) (final concentration 0.5 ng/mL), vortexed for 1 minute and centrifuged at 14,000 rcf for 10 minutes. The extracts were subject to solid phase extraction (SPE) clean up using Oasis MAX mixed mode polymeric SPE 96-well µElution Plate (p/n: 186001829). The final extract was eluted into Waters QuanRecovery Plates to minimize non-specific binding and maximize peptide recovery.
Semaglutide quantification was performed on an ACQUITY Premier UPLC System, with separations performed on 2.1 x 50 mm 1.7 µm ACQUITY Premier Peptide CSH C18 130 Å Column operated at 65 ºC and a flow rate of 0.4 mL/min. Following an initial hold of 0.3 minute at 30 % B the column was eluted with a linear reversed – phase gradient 30–65% B over the following 4.7 minutes, followed by a 2 minute wash at 95% B, the column was then re-equilibrated at 30% B for 2 minutes prior to the next injection.
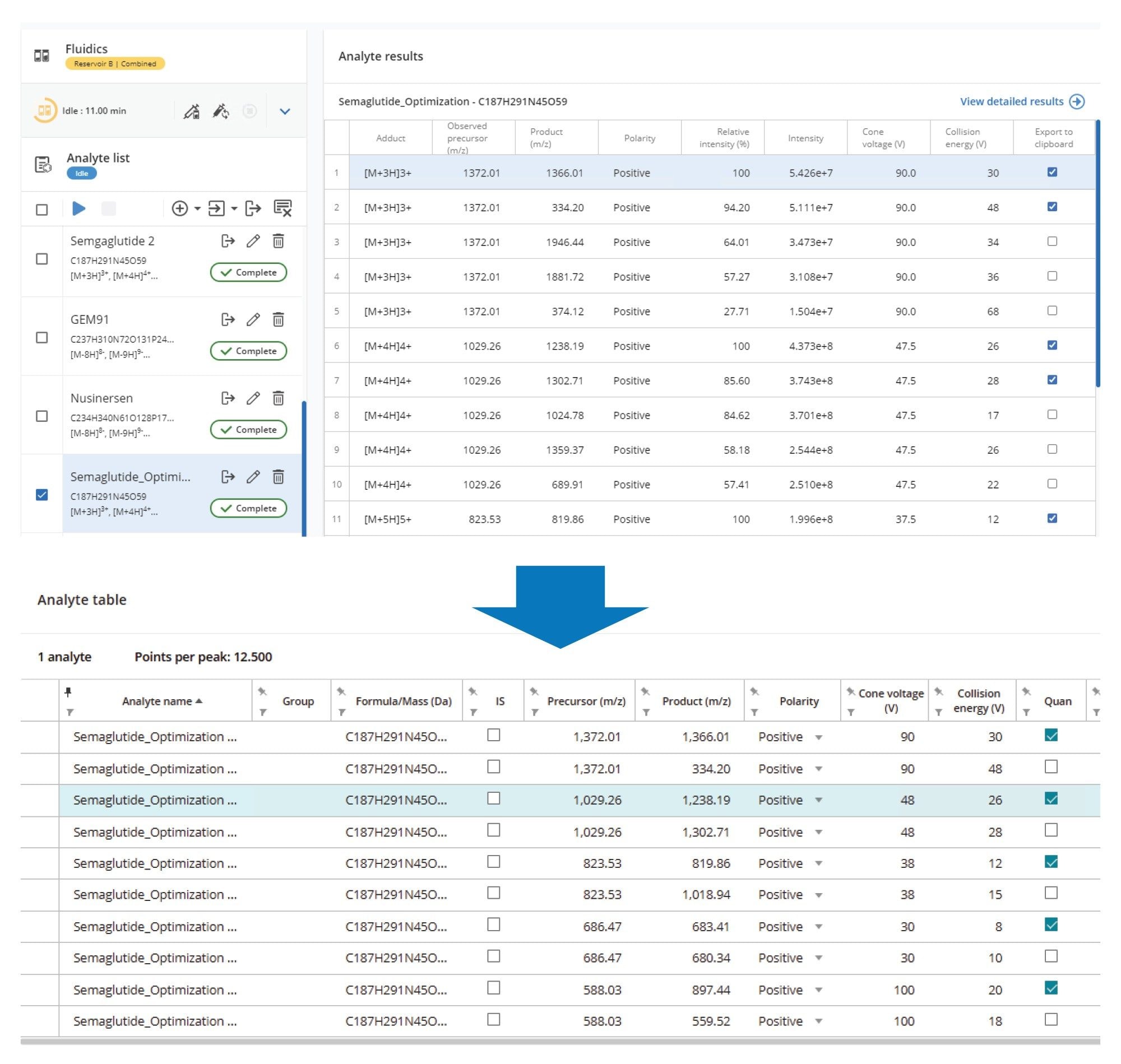
MRM transitions for semaglutide were optimized using the waters_connect Software Optimization tool via the input of the elemental composition of semaglutide (C187H291N45O59) and states M+3H3+, M+4H4+, M+5H5+, M+6H6+, M+7H7+ for evaluation. Transitions were further fine-tuned by injection and manual data review.
LC Conditions
|
LC system: |
ACQUITY Premier UPLC System |
|
Vials: |
QuanRecovery 96 well Plates (p/n: 186009184) |
|
Column(s): |
ACQUITY Premier Peptide CSH C18 Column, 130 Å, 1.7 µm, 2.1 x 50 mm (p/n: 186009460) |
|
Column temperature: |
65 °C |
|
Sample temperature: |
8 °C |
|
Injection volume: |
10 µL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
0.1% formic acid (aq) |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
Gradient Table

MS Conditions
|
MS system: |
Xevo TQ Absolute System |
|
Ionization mode: |
ES+ |
|
Capillary voltage: |
2.0 kV |
|
Desolvation temperature (ºC): |
600 |
|
Desolvation gas flow (L/Hr): |
1000 |

Data Management
|
Informatics: |
waters_connect for Quantification Software v 2.3 |
Results and Discussion
The quantification of peptides using LC-MS/MS for bioanalysis assays requires the development of SPE and chromatography method to separate the peptide of interest from the endogenous components in a biofluid and a selective MRM MS method (the workflow employed in this study is shown in Figure 2). Unlike small molecules, which largely generate singly charged precursor ions in electrospray MS, peptides typically give rise to several multiply charged precursor ions. The formation of several multiply charged ions (+3, +4, +5, +6 etc.,) combined with the generation of multiple fragment ions, adds complexity. Additionally, the requirement to select the best cone voltage and collision energy combination for each transition results in a large number of MRM transitions and energy combinations that all need to be evaluated and compared. This is a time consuming and laborious process, requiring the acquisition and comparison of potentially hundreds of MRM transitions. To simplify the MRM data acquisition and comparison process, the MRM Optimization tool within waters_connect Software, version 1.9, was employed. This tool automatically optimizes and evaluates the precursor ion à production ion collision energy pairs for the most sensitive MRM transition at each charge state. The list of potential transitions was transferred to waters_connect acquisition method editor (AME) where the viability of each MRM transition could be compared under LC conditions with extracted samples. The resulting raw data was then evaluated in waters_connect and the optimal MRM transition selected. The waters_connect MRM optimization and evaluation process is shown in Figure 3. A total of 25 precursor ion – product ion – collision energy – cone voltage combinations were evaluated with the optimal transition determined to be ESI +ve 1029.25à 1238, which corresponds to the [M-H]4+ ion.
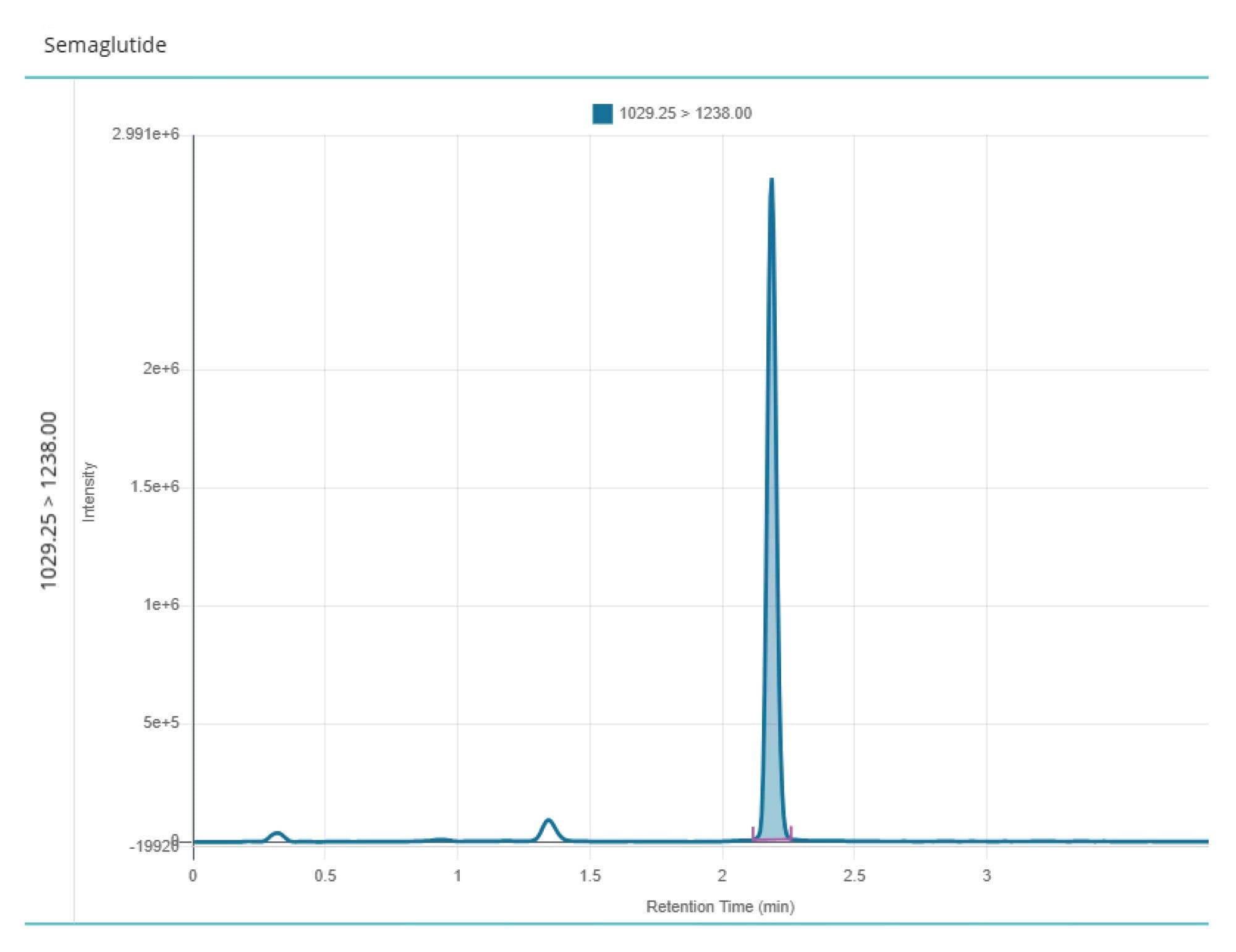
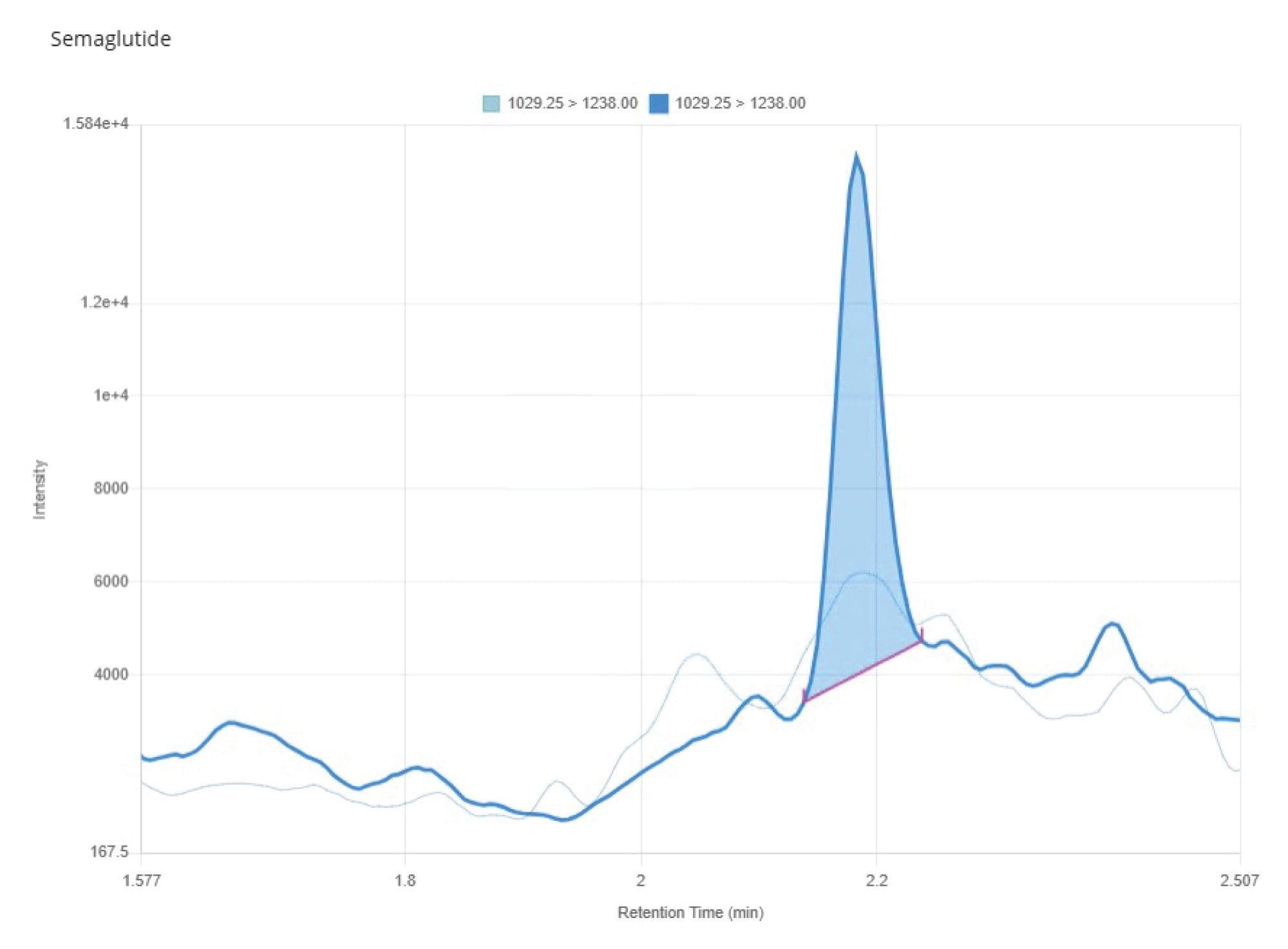
Both the liquid chromatography system and columns were constructed using HPS Technology5, which prevents non-specific binding of metal sensitive analytes such as peptides to commonly present metal components in the fluidic path column as well as the plastic surfaces of the SPE recovery plate. The synthetic peptide GLP-1 receptor agonist eluted from the chromatography column with a retention time of tR = 2.18 minutes, as shown in Figure 4. The optimized method demonstrated excellent peak shape for semaglutide with no evidence of non-specific binding impacting chromatographic performance and no observable carryover. The method, based on SPE, was found to be linear (R2 = 0.9985) over the calibration range 0.2–100 ng/mL using 1/x weighting, as shown in Figure 5. The signal to noise value (S/N) for the 0.2 ng/mL calibrator was greater than 5:1 when compared to a blank plasma sample extract, as shown in Figure 6, indicating that there was no detectable plasma matrix interference which could compromise assay specificity or limit of quantification.

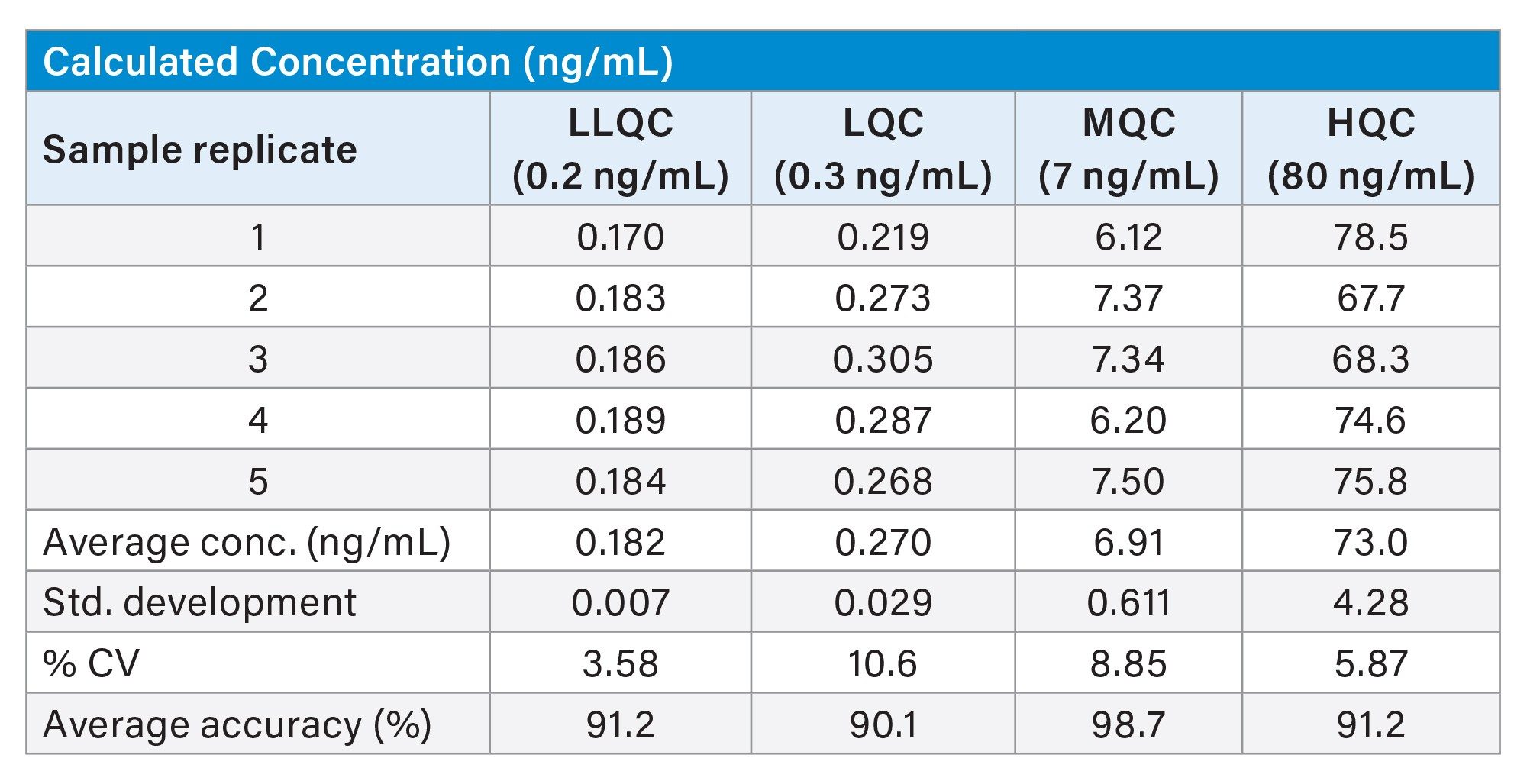
The repeatability and accuracy of quantification was assessed by the replicate preparation of QC samples which were interspersed throughout the sample analysis. The %CV and accuracy were <10.63 and >90%, respectively, and well within acceptable parameters for a discovery “fit for purpose” bioanalysis assay.
Conclusion
A sensitive and selective assay for the quantification of semaglutide in human plasma has been successfully developed using the Xevo TQ Absolute System with the ACQUITY Premier UPLC System and Oasis MAX SPE Plates. The described workflow provides analytical sensitivity down to 0.2 ng/mL for semaglutide. Excellent linearity was observed across the calibration range of 0.2–100 ng/mL and no analyte carryover or interference was observed in the blank plasma samples. Repeatability of the method was <4% at the LLQC level and accuracy of quantification was >90%, well within acceptable limits for a discovery “fit for purpose” bioanalytical assay. The waters_connect MRM Optimization tool was used to simplify the MS MRM method development process allowing for greater than 25 combinations of precursor ion, product ion, collision energy, and cone voltages to be rapidly evaluated and the optimal combination selected. The use of ACQUITY Premier UPLC Systems, MaxPeak UPLC Columns, and QuanRecovery Plates mitigated analyte absorption to the metal and plastic surfaces of the chromatography system/columns and sample plates, resulting in symmetrical LC peak shapes and minimal analyte loss.
References
- Wang L, Wang N, Zhang W, Cheng X, Yan Z, Shao G, Wang X, Wang R & Fu C. Therapeutic peptides: current applications and future directions. Sig Transduct Target Ther. 2022; 7(48). https://doi.org/10.1038/s41392-022-00904-4
- Nauck M A, Meier J J. Pioneering oral peptide therapy for patients with type a. Diabetes. Lancet Diabetes Endocrinol. 2019; 7: 500–502.
- Overgaard RV, Delff PH, Petri KCC, Anderson TW, Flint A, Ingwersen SH. Population Pharmacokinetics of Semaglutide for Type 2 Diabetes. Diabetes Ther. 2019 Apr;10(2):649-662. doi: 10.1007/s13300-019-0581-y.
- Bracchiglione, J., Meza, N., Franco, J. V., Escobar Liquitay, C. M., Novik A, V., Ocara Vargas, M., Lazcano, G., Poloni, D., Rinaldi Langlotz, F., Roqué-Figuls, M., Munoz, S. R., and Madrid, E. (2025). Semaglutide for adults living with obesity. The Cochrane database of systematic reviews, 10(10), CD015092. https://doi.org/10.1002/14651858.CD015092.pub2
- M. DeLano, T. H. Walter, M. A. Lauber, M. Gilar, M. C. Jung, J. M. Nguyen, et al. Using Hybrid Organic–Inorganic Surface Technology to Mitigate Analyte Interactions with Metal Surfaces in UHPLC. Anal. Chem. 2021 (93) 14, 5773-57. DOI: 10.1021/acs.analchem.0c05203.
720009121, November 2025