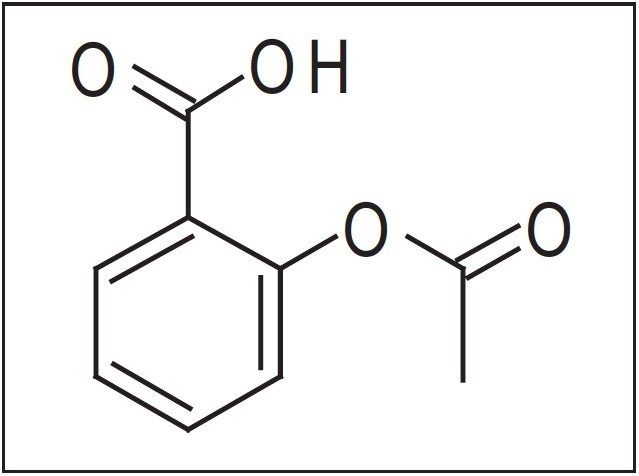
This application note describes the small-scale purification of a synthesized drug product, acetylsalicylic acid, using preparative-scale liquid chromatography.
During the drug discovery process, organic compounds are often synthesized and then isolated from reaction mixtures. These isolated compounds are seldom pure – they are usually contaminated with reaction precursors, small amounts of similar compounds, and reaction by-products formed during the reaction.
In order to characterize these compounds or use them for other purposes, it is necessary to purify them. The purification process may use any number of techniques (liquid/liquid extraction or recrystallization, for example). These techniques are often slow and not easily automated.
Liquid chromatography can be used to purify compounds from these reaction mixtures if classical techniques are not entirely successful, not desirable, or if a high level of purity is desired.
This application note describes the small-scale purification of a synthesized drug product, acetylsalicylic acid, Figure 1, using preparative-scale liquid chromatography. A simple technique for determining suitable separation conditions will also be described.
|
LC system: |
Alliance HPLC System |
|
Detector: |
PDA Detector |
|
Column: |
XTerra RP18, 4.6 x 100 mm, 5 μm |
|
Column temp.: |
Ambient |
|
Flow rate: |
1.5 mL/min |
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Acetonitrile |
|
Mobile phase C: |
2% Formic acid in water |
|
Data collection: |
Empower 2 Software |
|
LC system: |
Waters Purification System |
|
Pump: |
2545 Quaternary Gradient Module |
|
Injector: |
FlexInject, 10 mL loop |
|
Collector: |
Fraction Collector III |
|
Detector: |
2489 UV/Vis @ 280 nm (semi-prep flow cell) |
|
Column: |
XTerra Prep, 30 x 150 mm, 5μm |
|
Column temp.: |
Ambient |
|
Flow rate: |
64 mL/min |
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Acetonitrile |
|
Mobile phase C: |
2% Formic acid in water |
|
Data collection: |
MassLynx Software with FractionLynx Application Manager |
The preparative chromatography system (Figure 2) consisted of the 2545 Quaternary Gradient Module, a low-pressure mixing solvent delivery module capable of flow rates up to 150 mL/min; the 2489 UV/Visible Detector; the Fraction Collector III; and the FlexInject Manual Dual Injector Module.

The system was controlled using MassLynx Software with the FractionLynx Application Manager. FractionLynx controls fraction collection triggering, tracks samples, fractions, and associated data through its easy-to-use browser.
This preparative LC system configuration is designed to purify a few fractions a day, and, since Waters’ versatile purification systems are upgradeable, the system can easily be expanded as laboratory workloads increase.
Acetic anhydride (1.5 mL) was added to 1.0 grams of salicylic acid along with one drop of concentrated sulfuric acid. The entire mixture was placed in a water bath at 55 °C for 30 min with occasional stirring. The mixture was cooled to room temperature and the resulting crystals washed with water (~ 200 mL). The washed crystals were dissolved in 2.0 mL of dimethyl sulfoxide, of which 5.0 μL of were removed and diluted to 1.0 mL in methanol; this solution was used for HPLC method development. The remaining DMSO solution was set aside for preparatory HPLC.
To determine the optimal separation conditions for the preparative purification, a series of four analytical separation scouting runs were performed (Figure 3). Each gradient separation used the same starting conditions (85% A, 10% B, and 5% C) and the same gradient time (10 min). Organic solvent content was varied in each of the four runs over the gradient time:
Run 1, 10% B to 90% B
Run 2, 10% B to 75% B
Run 3, 10% B to 50% B
Run 4, 10% B to 25% B
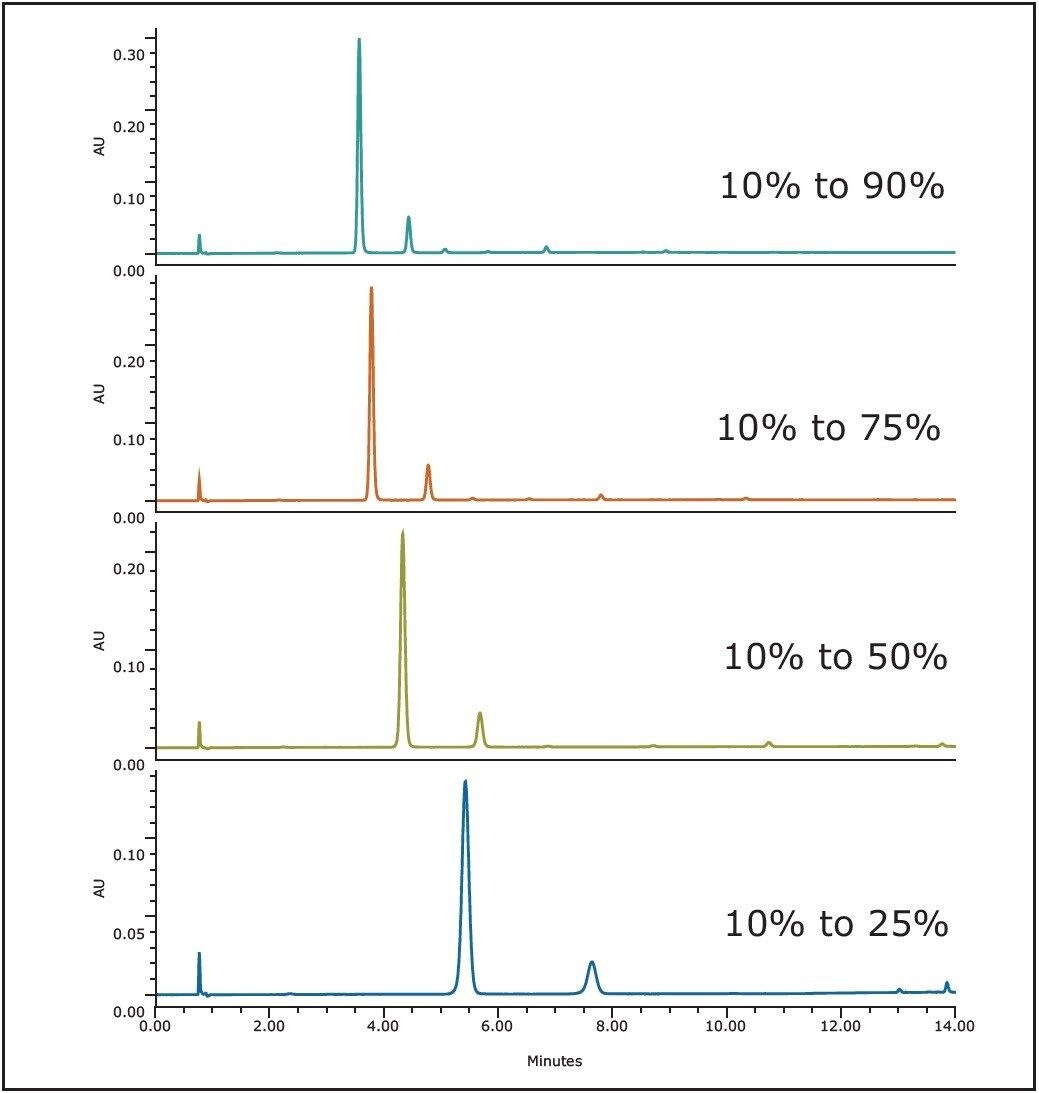
Comparison of the four chromatograms (Figure 3) shows that the fourth run provided maximum resolution for the two main peaks in the mixture, allowing for higher sample loads and ultimately higher yields. Based on these runs, calculations showed that the crude mixture had an approximate purity of 74%, based on UV area %. In cases where the four scouting runs do not provide suitable resolution, data from those runs could be modeled using chromatography modeling software such as Molnar-Institute’s DryLab. This data was also used to determine the experiment’s optimal detection wavelength, which was determined to be 280 nm.
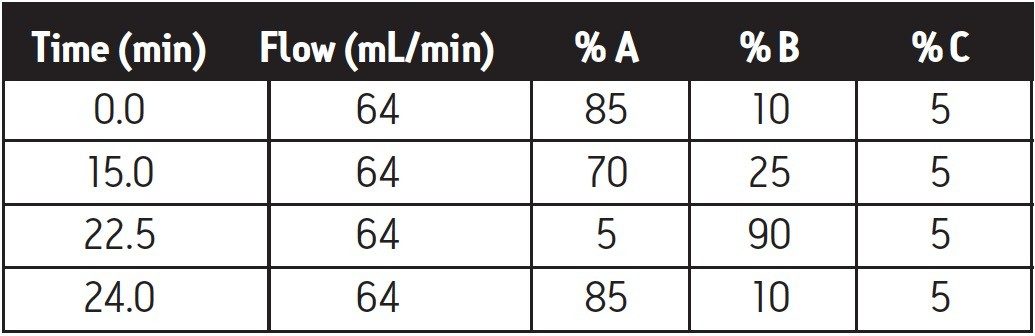
The analytical method was scaled to preparatory using the Basic Gradient Scaler function of the Waters Prep Calculator (Figure 4), generating a gradient table appropriate for the 30 x 150 mm preparatory column (Table 1). The fraction collector was set up to receive the collected fractions into flasks. Utilizing the FractionLynx Application Manager, fraction collection was triggered based on UV (280 nm) signal, and subsequently stopped when the UV absorbance reached 0.13 AU. These values can be adjusted as required.
The entire solution generated from the synthesis was injected onto the preparatory column (Figure 5). The target peak (Peak 1) was collected from 4.5 to 7.6 minutes. Peak 2 was known to be salicylic acid and was discarded post-collection. Immediately following collection, a small portion of the peak 1 fraction was removed and analyzed for purity (Figure 6). The purity of that collected fraction, based on UV area %, was calculated to be >99.9%. The fraction was dried down and yielded 526 mg of crystalline material. As a final confirmatory check, a small portion of the purified crystals were analyzed and found to have a purity of >99.5%.
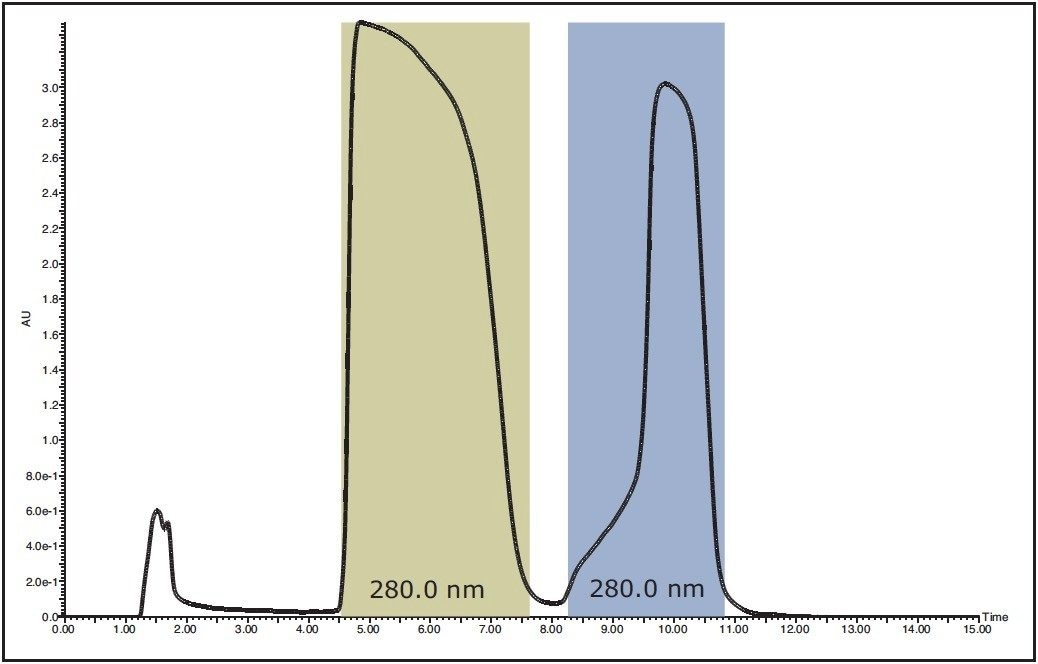
Figure 5. UV-directed purification of organic synthesis mixture.
The shaded area represents the collected peak fractions.
720002814, October 2008