Reliable Purification of Full-Length Product and Enhanced Isolation and Detection of Impurities With Ready to Use XBridge Premier Oligonucleotide BEH™ C18 OBD™ Preparative Columns
Michael Miller1, Paul Lefebvre1, Makda Araya2, Balasubrahmanyam Addepalli2, Mandana Fasth3, Christian Reidy2, Matthew Lauber2
1 Neopharm Boston, Milford, MA, USA
2 Waters Corporation, United States
3 Waters Corporation, Stockholm, Sweden
Published on April 30, 2025
Abstract
Synthetic oligonucleotide therapeutics require reliable purification techniques so that impurities can be carefully collected and submitted for toxicology studies. In this work, we evaluated MaxPeak™ High Performance Surfaces (HPS™) technology hardware as applied to preparative ion-pair reversed-phase liquid chromatography (IP-RPLC). Compared to traditional stainless-steel columns, HPS-enabled preparative columns demonstrated improved consistency and recovery of early-eluting impurities. This performance advantage can provide assurances for more robust impurity profiling and minimizes concerns related to sample loss or changes in chromatography during testing. Moreover, the availability of MaxPeak HPS-equipped optimal bed density (OBD) Premier preparative columns provides more predictable scaling of the analytical chromatography already being performed by oligonucleotide laboratories using ACQUITY™ Premier Column technology.
Benefits
- Batch tested and selected XBridge Oligonucleotide BEH130 C18 5 µm 130 Å Sorbent Particles provide predictable, low secondary interaction separations by ion pair reversed phase chromatography (IP-RP)
- MaxPeak HPS hardware components minimize non-specific adsorption of oligonucleotides and improve recovery of early eluting sample components
- Construction with a corrosion resistance hardware design can provide long-lived performance of columns
- The XBridge MaxPeak Premier OBD preparative column provides direct and predictable scale-up from pre-purification assessments using MaxPeak Premier analytical columns
Introduction
Synthetic oligonucleotide therapeutics, including antisense oligonucleotides, small interfering RNAs (siRNAs), and aptamers, represent a growing class of precision medicines targeting genetic disorders and diseases. These molecules are chemically synthesized and inherently complex, often accompanied by impurities such as truncated sequences, deprotected intermediates, or chemically modified variants. For pharmaceutical companies, understanding and controlling these impurities is critical for ensuring drug safety and efficacy. Regulatory agencies mandate toxicology studies that evaluate the potential effects of known impurities at relevant concentrations. This requires isolating and purifying these impurities with high precision to study their biological impact. Advanced purification techniques like ion-pair reversed-phase (IP-RP) HPLC enable the separation and characterization of these impurities, providing the high-purity materials needed for toxicological assessments and meeting regulatory standards. This rigorous approach ensures that oligonucleotide therapeutics are both safe and effective for clinical use.
In an IP-RP approach, oligonucleotides are separated based on their hydrophobic interactions with the stationary phase, which are enhanced by ion-pairing agents such as triethylammonium acetate (TEAA). These agents form temporary complexes with the negatively charged phosphate backbone of the oligonucleotides, allowing them to interact more effectively with the hydrophobic column surface. The separation occurs as a function of the oligonucleotide’s length, base composition, and hydrophobicity, with gradient elution using water and organic solvents like acetonitrile or methanol. Despite the effectiveness of tried-and-true ion pairing interactions, oligonucleotides and their impurities can present challenges to a purification scientist. Target impurities with anionic functionality can adsorb to electropositive metal surfaces, which can lead to incomplete recoveries and bias results upon fraction collection. One approach to mitigate sample loss is to condition the flow path with a series of sample injections until the expected signal response is observed. A passivated state may be achieved more quickly with a higher sample loading. As noted in the MISER study by Gilar et al., the surface passivation effect is not permanent, and de-passivation may occur overtime in the mobile phase. This phenomenon of passivation and desorption can lead to higher injection to injection variability over the lifetime of the column.
To address this challenge, Waters is introducing alternative hardware components to lab scale purification HPLC columns, namely MaxPeak High Performance Surfaces (HPS). MaxPeak HPS hardware components are manufactured with a vapored deposited organosilica protective layer and are a transformative innovation in oligonucleotide chromatography, addressing a critical challenge in the analysis and purification of synthetic oligonucleotides. Traditional stainless steel column hardware can cause unwanted interactions between the metal surface and the negatively charged phosphate backbone of oligonucleotides, leading to sample adsorption, poor peak shape, and inconsistent recovery. MaxPeak HPS effectively mitigates these interactions by providing a chemically inert and non-reactive surface, thereby circumventing the need for surface passivation with sacrificial injections. This can yield greater retention consistency, improved peak resolution, and higher recoveries out of the box. By minimizing metal-ion interactions, MaxPeak HPS column hardware components can facilitate more reliable, more reproducible, and higher quality chromatographic results, crucial for both analytical and preparative applications in oligonucleotide development.
In this application note, we explore the initial use of a 10 mm ID oligonucleotide IP-RPLC column in a scenario mimicking a situation in which an oligonucleotide synthesis and purification lab needs to collect fractions for toxicology studies. A crude preparation of 20-mer oligonucleotide is fractionated into 2 min intervals. A column built with HPS components is compared to a standard metal hardware version. Example preparative mass loads are compared with a focus on the recovery of early eluting components. Analytical runs for purity and composition analysis of example fractions are also studied. Results confirm that the initial use of a column without MaxPeak HPS technology can require passivation and produce biased recoveries of early eluting impurities, which can be misleading to purification scientists and lead to biased toxicology and tolerability studies.
Experimental
Sample Preparation
Crude oligonucleotide was dissolved in Mobile Phase A to a concentration of 10 mg/mL. The sample was heated at 95 °C for 5 minutes then transferred to storage at 4 °C. Aliquots used for purification were allowed to come to room temperature prior to injection.
Analytical IP-RPLC-UV/MS samples were prepared from fractions collected in 15 mL conical centrifuge tubes and stored at -20 °C. Fully thawed fractions were vortexed for at least 5 seconds and inverted at least 10 times before sampling. A 500 µL volume was transferred to low adsorption microfuge tubes and dried by centrifugal evaporation. Dried fractions were reconstituted with 50 µL of 0.1% DIPEA and 1% IonHance™ HFIP (p/n: 186010781) in 18.2 MΩ*cm water (MPA), vortexed for at least 5 seconds and transferred to QuanRecovery™ with MaxPeak HPS 12 x 32 mm Polypropylene 300 µL Screw Cap Vials (p/n: 186009186) with Blue 12 x 32 mm Screw Neck Cap and Pre-slit PTFE/Silicone Septum (p/n: 186000305). A 10 µL volume was injected onto an ACQUITY Premier Oligonucleotide BEH C18 300 Å 1.7 µm, 2.1 x 50mm Column (p/n: 186010539).
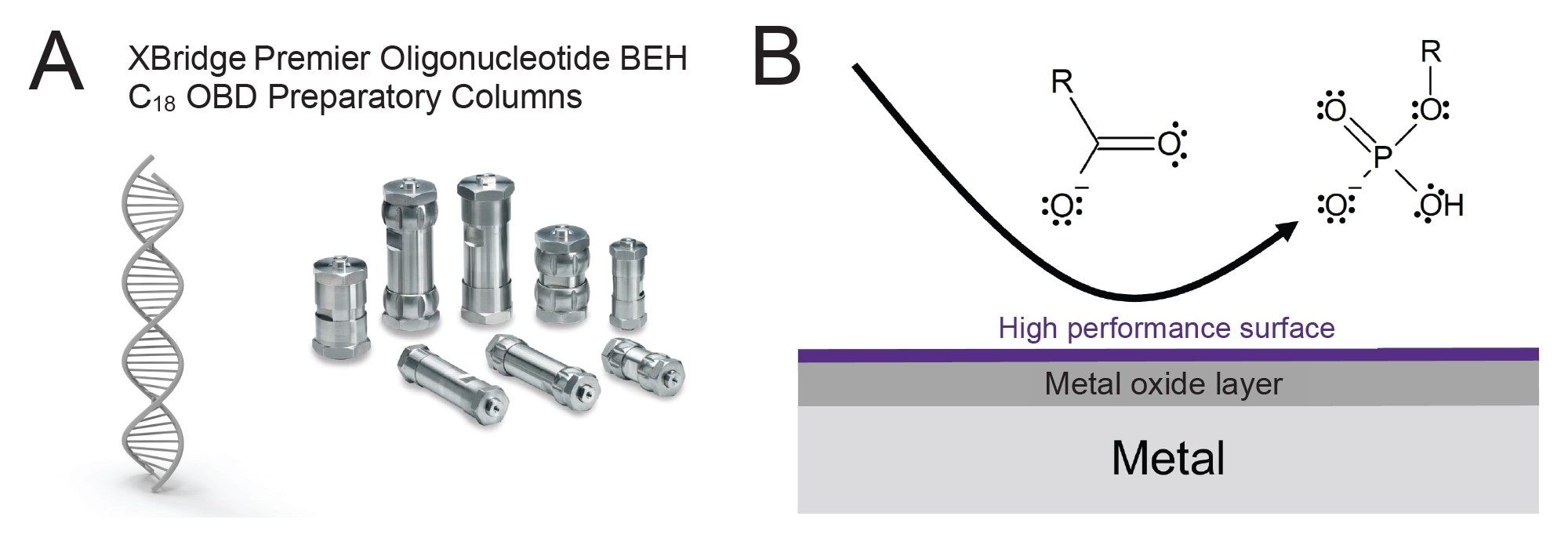
Column Technology Considerations
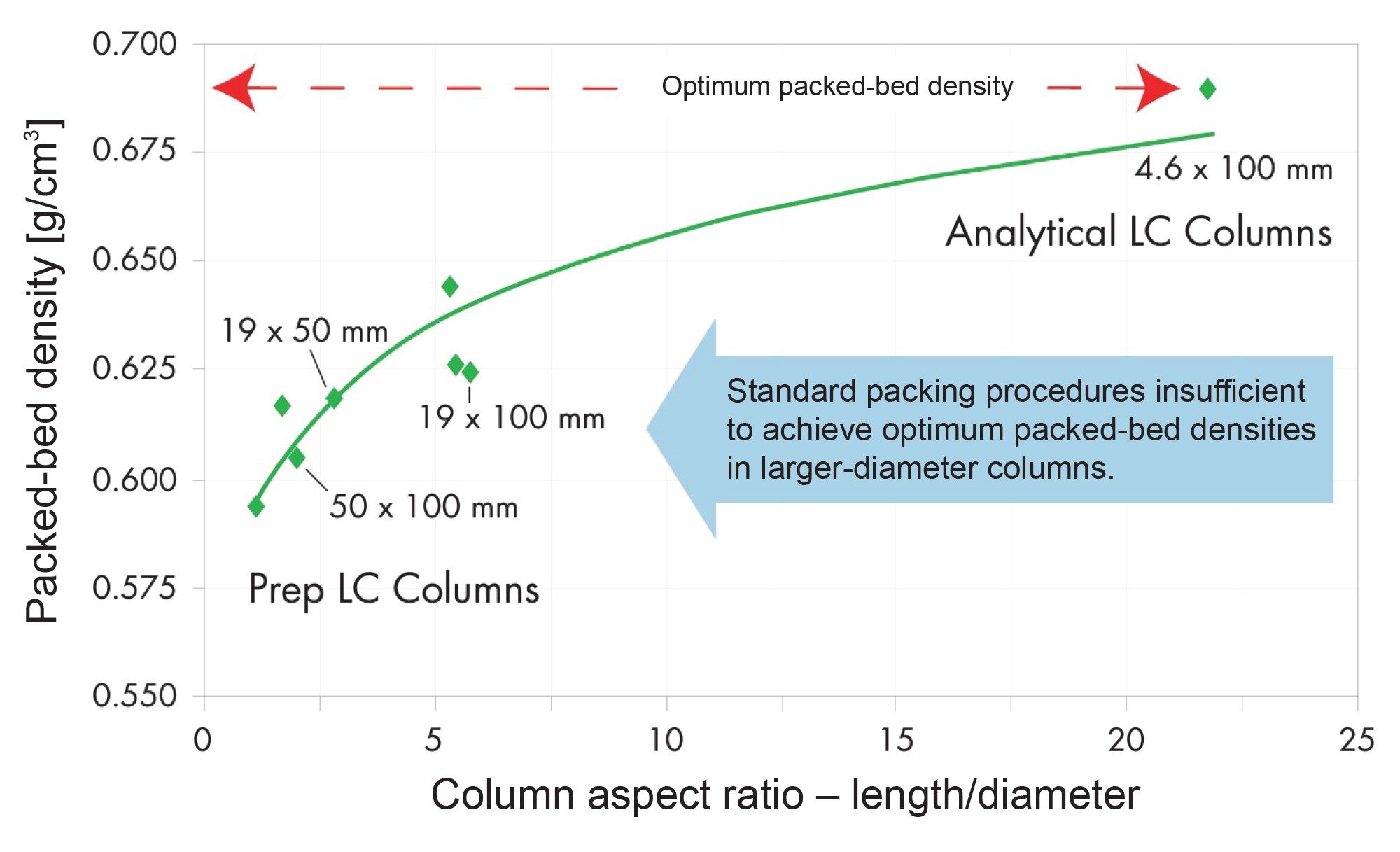
In addition to featuring MaxPeak HPS components, XBridge Premier OBD Preparative Columns are made using an optimum bed density (OBD) packing process that combines high-pressure slurry packing with calculated axial compression, particularly addressing the less-dense inlet end of the column bed. This method ensures a uniform and stable bed density throughout the column, effectively eliminating voids and preventing channeling (Figure 2). The result is enhanced mechanical stability, leading to extended column lifetimes and consistent chromatographic performance, even under the high-pressure conditions typical in preparative HPLC.
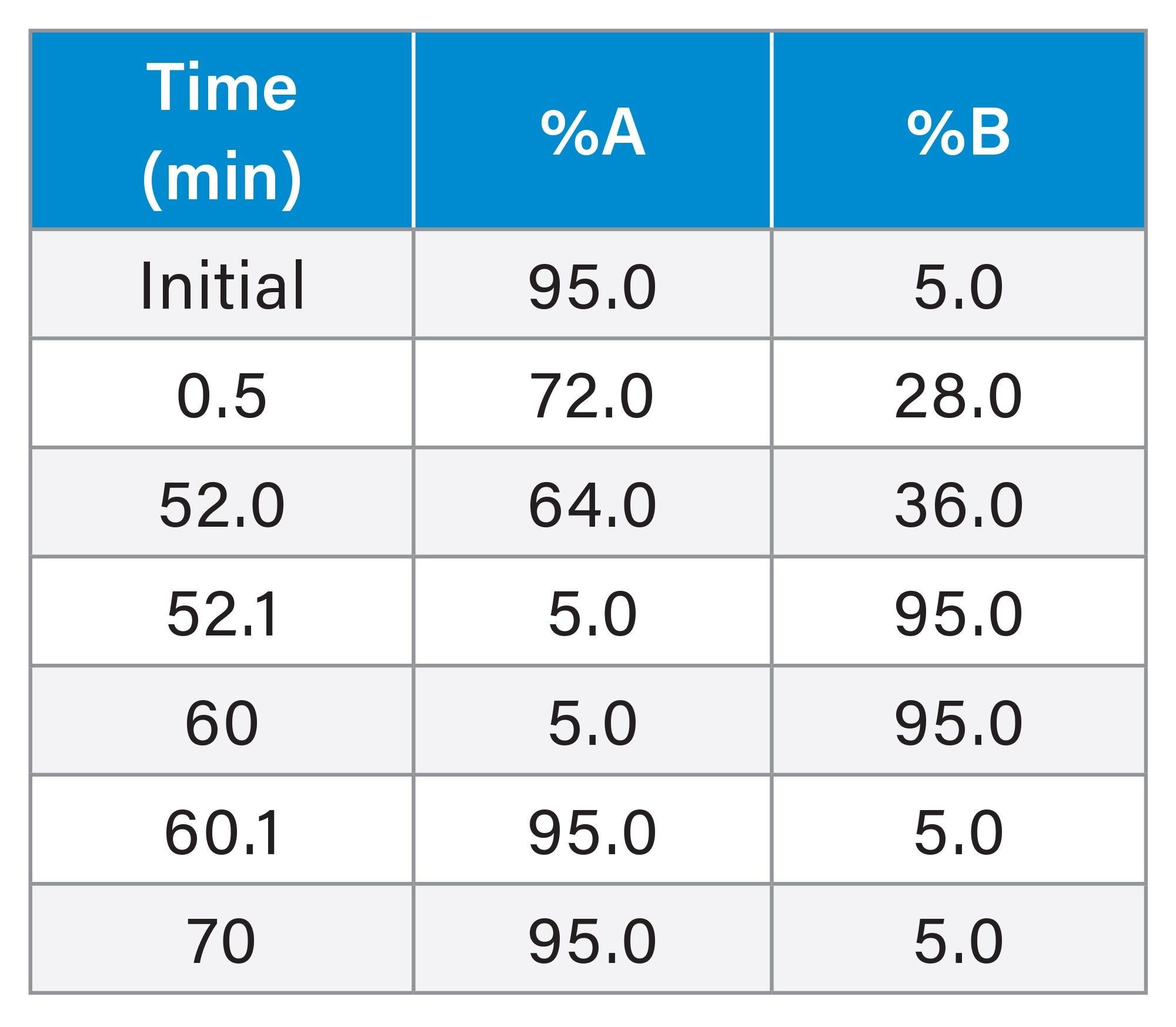
Purification IP-RPLC
|
LC system: |
A Waters Analytical Scale Purification System was employed to perform 10 mm ID semi-preparative chromatography. The outlet flow paths of two Arc HPLC Quaternary Solvent Manager-R pumps were combined and used in combination with a 3767 Sample Manager, CM--30S Column Manager, and 2489 UV/Vis Detector. |
|
UV wavelength: |
254 nm |
|
Data acquisition: |
MassLynx™ Mass Spectrometry Software with FractionLynx application manager |
|
Column: |
XBridge Premier Oligonucleotide BEH C18 OBD Preparative Column 130 Å, 5 µm, 10 mm x 150 mm (p/n:186011208) XBridge Oligonucleotide BEH C18 OBD Preparative Column 130 Å, 5µm, 10 mm x 150 mm (p/n: 186011153) |
|
Column temperarure: |
60 °C |
|
Sample temperature: |
Room Temperature |
|
Vials: |
15 mL conical centrifuge tube |
|
Injection volume: |
50 and 500 µL |
|
Flow rate: |
4.5 mL/min |
|
Mobile phase A: |
100 mM Triethylammonium acetate (TEAA), pH 7.0 water |
|
Mobile phase B: |
20:80 Mobile Phase A: Acetonitrile |
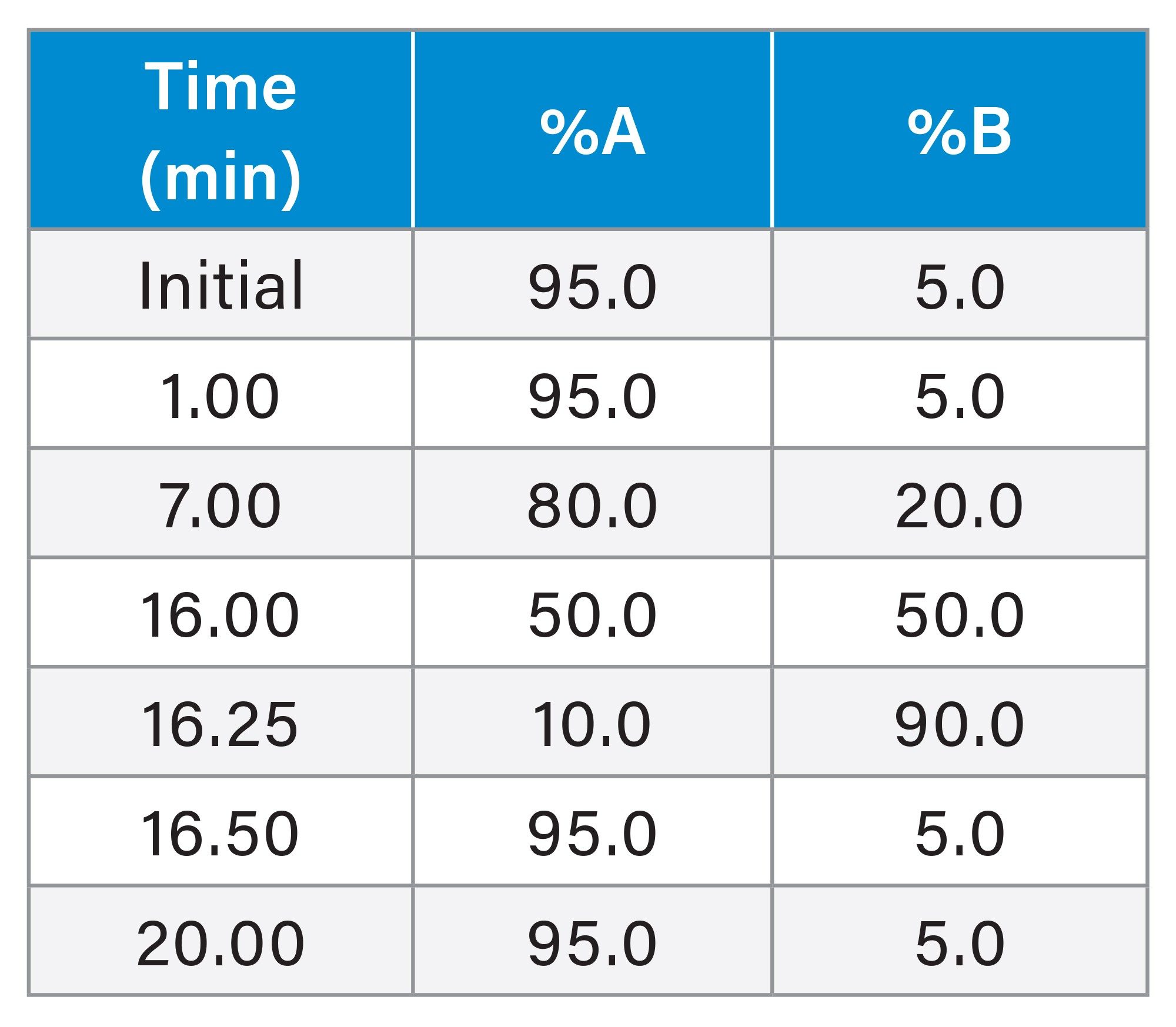
Analytical IP-RPLC-UV/MS
|
LC system: |
ACQUITY UPLC™ I-Class PLUS System with RDa Detector |
|
Wavelength: |
260 nm, 20 points/sec |
|
Data Acquisition: |
waters_connect™ Software |
|
Column: |
ACQUITY Premier Oligonucleotide BEH C18 Column, 300 Å, 1.7µm, 2.1 x 50 mm (p/n: 186010539) |
|
Sample temperature: |
5 °C |
|
Column temperature: |
70 °C |
|
Injection volume: |
10 µL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
0.1% N,N-diisopropylethylamine (DIPEA) and 1% IonHance HFIP (p/n: 186010781) in 18.2 MΩ*cm water |
|
Mobile phase B: |
0.0375% DIPEA and 0.075% IonHance HFIP (p/n: 186010781) in 65:35 Acetonitrile: 18.2 MΩ*cm water |
|
MS mode: |
Full scan with fragmentation |
|
Mass range: |
High (400 to 5000 m/z) |
|
Polarity: |
Negative |
|
Scan rate: |
2 Hz |
|
Cone voltage: |
40 V |
|
Fragmentation cone voltage: |
80–200 V |
|
Capillary voltage: |
0.80 kV |
|
Desolvation temperature: |
400 °C |
Results and Discussion
Testing the Effects of MaxPeak High Performance Surfaces (HPS) in Oligonucleotide Preparative Chromatography
A head-to-head comparison between an XBridge Oligonucleotide BEH C18 5 µm 130 Å OBD Preparative Column and an XBridge Premier Oligonucleotide BEH C18 5 µm 130 Å OBD Preparative Column was performed using a 20-mer synthetic oligonucleotide (0.5 mg mass load) and an ion-pair reversed phase (IP-RP) mode purification. The XBridge Premier Oligonucleotide OBD Preparative Column exhibited better recoveries and a more stable elution profile across subsequent injections. In comparison, the same XBridge Oligonucleotide BEH C18 particles packed in traditional steel hardware displayed a less consistent profile and a higher variability between injections. This can potentially limit its suitability for detailed impurity characterization and is an example of variation observed with column conditioning. A zoomed view and overlay of these particular differences in back-to-back injection performance are provided in the form of overlaid chromatograms in Figure 3. The overlay demonstrates that some peaks are reproducible between column types, while other peaks are only observed on the MaxPeak HPS OBD Column. This is especially clear in the early part of the run, in the 8.7–14.7 minute window, where the most polar components are eluting. However, this phenomenon is still observed later in the run with undetected peaks on the stainless steel column between 21.0 and 26.7 minutes.
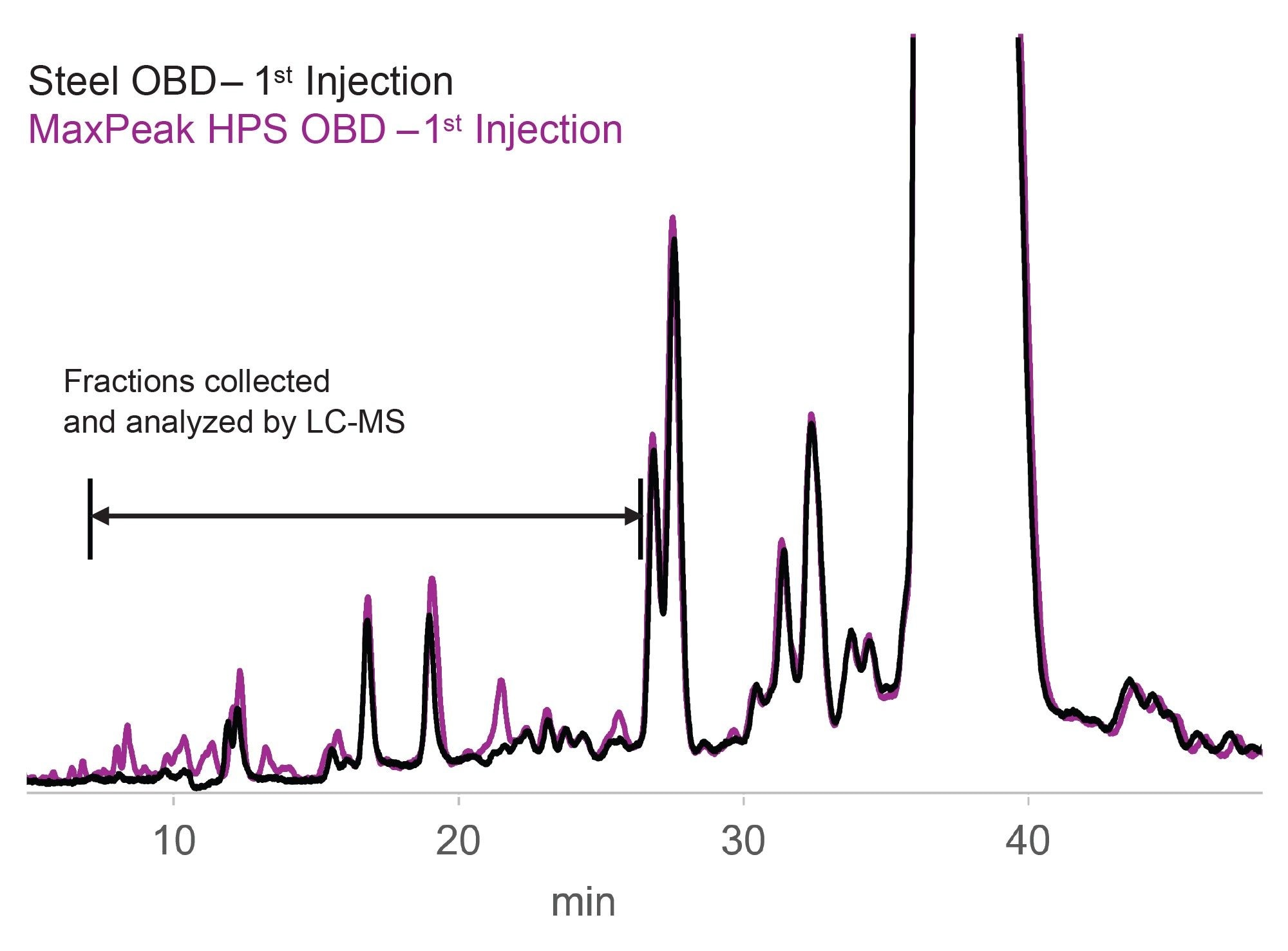
Analysis of Fractions using IP-RPLC-UV/MS
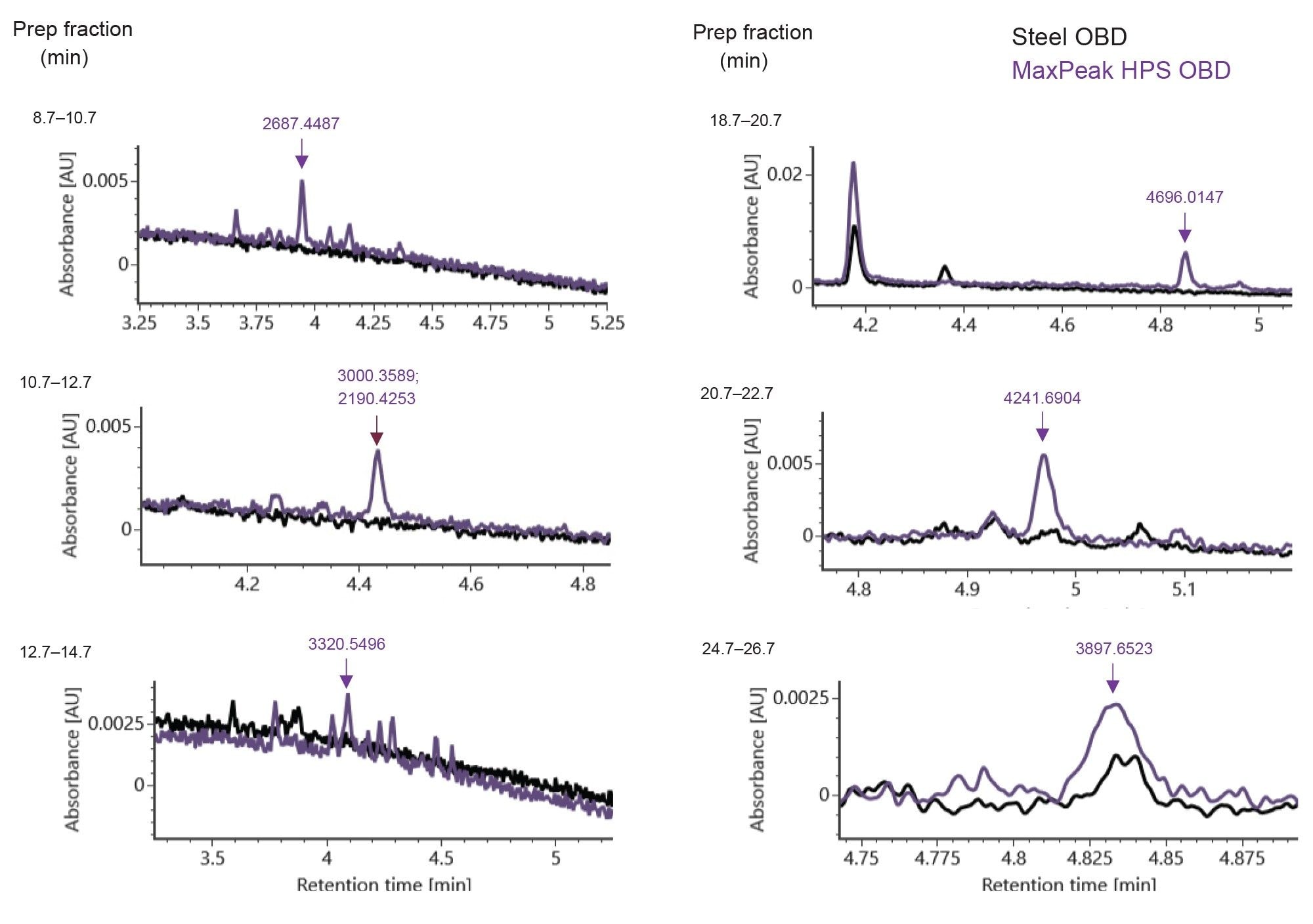
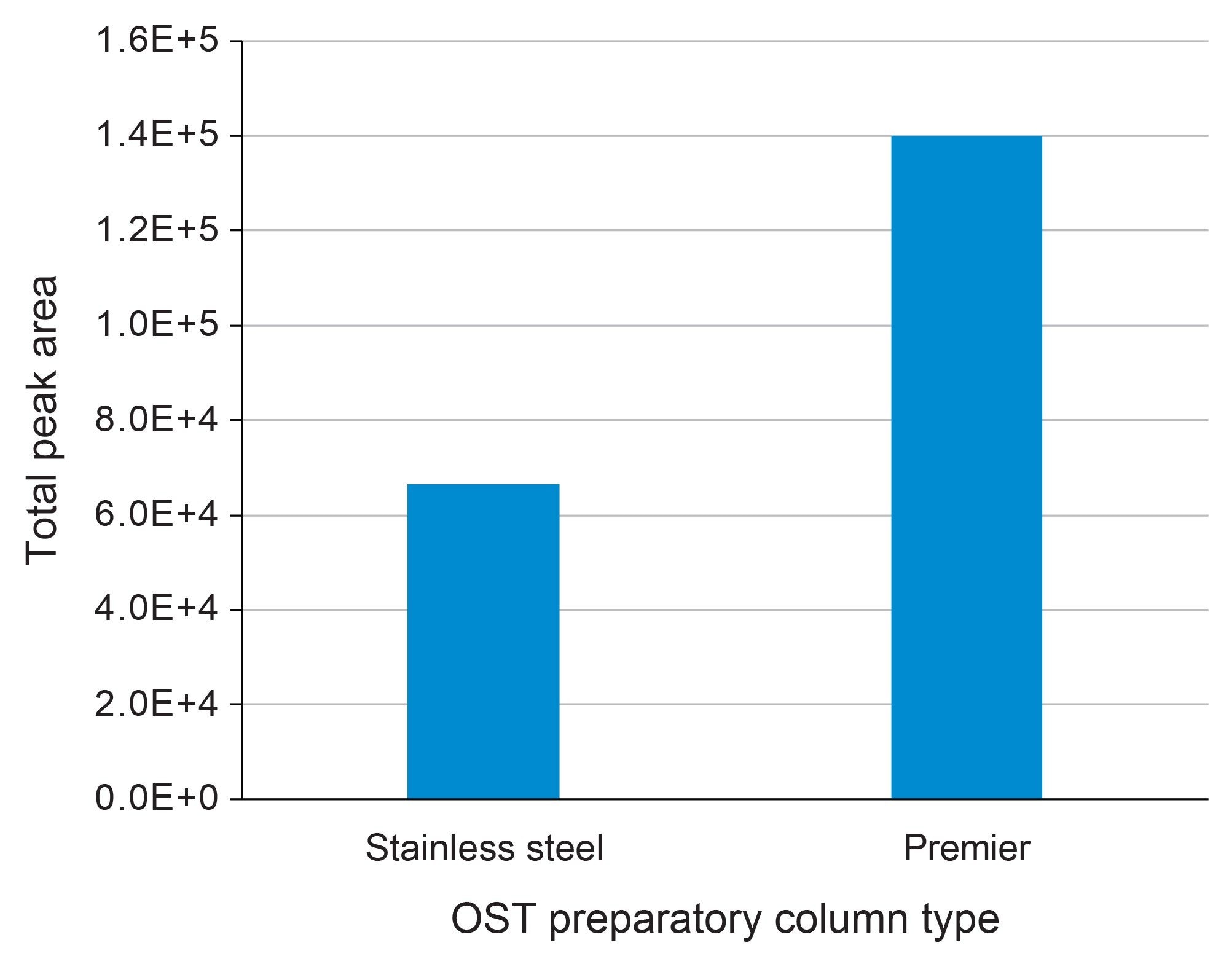
Fraction collection of the early eluting peaks in 2-minute windows from 8.7 to 26.7 minutes for both preparative methods was performed to further investigate the nature of the impurities. Each 2-minute fraction was evaporated to dryness and reconstituted in 50µL MPA (0.1% DIPEA and 1% IonHance HFIP in 18.2 MΩ*cm water) and analyzed by IP-RPLC-UV/MS. The analytical scale UV chromatograms can be readily compared in Figure 4. The MaxPeak HPS Prep Column’s improved recovery attributes by more than 100% higher peak area for impurity peaks detected in the analytical method when compared to the stainless-steel equivalent as compared in Figure 5. The TUV of the various collection windows (8.7 to 10.7, 10.7–12.7, 18.7–20.7, 20.7–22.7, and 24.7–26.7 minutes) was able to detect a series of impurity peaks not recovered from the stainless-steel purification method.
Online LC-MS mass analysis provided further information about the collected fractions. To simplify the comparison, the raw MS data from each fraction was deconvoluted and the unique mass values that were observed only on the MaxPeak HPS Premier OBD column are highlighted in Figure 4. It is suspected that these compounds were lost during purification in the stainless steel OBD Column due to non-specific adsorption and therefore were not recovered. It is also interesting to note that the impurity peaks detected from both columns exhibited higher total signal from the MaxPeak HPS Premier OBD fractions (Figure 5). This data supports the superiority of MaxPeak HPS Premier OBD prep Columns over stainless-steel prep column for improved detection and quantification of oligonucleotide impurities.
Conclusion
This study shows how advanced technologies such as Waters MaxPeak High Performance Surfaces (HPS) can be applied even in oligonucleotide preparative columns to overcome longstanding challenges in the purification of synthetic oligonucleotides. As demonstrated, traditional stainless-steel hardware can introduce unwanted interactions that compromise the recovery of early-eluting impurities most especially from initial runs, which can potentially lead to biased toxicology studies and unreliable data. MaxPeak HPS, with its inert, organosilica-protected surfaces, mitigates these issues by assuring more consistent retention and higher recoveries, starting with the column’s very first injection.
For laboratories conducting oligonucleotide synthesis and purification, particularly for toxicology studies, the use of HPS-enabled columns is strongly recommended to ensure accurate fraction collection and reliable impurity profiling. When initiating purification workflows, scientists should consider transitioning to HPS-based columns to avoid passivation procedures and to reduce the risk of biased recoveries. Moreover, adopting analytical tools compatible with these same innovations will enhance the reliability of purity and composition analysis. By integrating HPS technology into both preparative and analytical workflows, labs can more reliably achieve desired, accurate results, ultimately advancing the development of safe and effective oligonucleotide therapeutics.
Integration of Purification and Toxicity Studies: The synergy between purification and toxicity studies is foundational in the lifecycle of readying and finalizing oligonucleotide drug filings. This study underscores the importance of column selection in oligonucleotide purification workflows, particularly when early eluting impurities play a pivotal role in safety evaluations ensuring that observed therapeutic effects are due to the molecule itself rather than contaminants and that no adverse effects come from the dosing of any known impurities. Together these processes ensure safety and efficacy of the intended drug.
References
- Villiger, L., Joung, J., Koblan, L. et al. CRISPR technologies for genome, epigenome and transcriptome editing. Nat Rev Mol Cell Biol 25, 464–487 (2024) https://doi.org/10.1038/s41580-023-00697-6.
- Nakamura, M., Gao, Y., Dominguez, A.A. et al. CRISPR technologies for precise epigenome editing. Nat Cell Biol 23, 11–22 (2021). https://doi.org/10.1038/s41556-020-00620-7.
- Minkner R, Boonyakida J, Park EY, Wätzig H. Oligonucleotide separation techniques for purification and analysis: What can we learn for today'stasks?. Electrophoresis. 2022; 43: 2402–2427. https://doi.org/10.1002/elps.202200079.
- Gilar, M., DeLano, M., and Gritti, F. (2021). Mitigation of analyte loss on metal surfaces in liquid chromatography. Journal of Chromatography A, 1650, 462247. https://doi.org/10.1016/j.chroma.2021.462247.
720008749, April 2025