Analysis of Underivatized Bisphosphonate Drugs Using HILIC-MS
Kenneth Berthelette, Melissa Aiello, Chris Collins, Thomas H. Walter
Waters Corporation, United States
Published on June 04, 2025
Abstract
Bisphosphonate drugs are an important class of therapeutics used for the treatment of osteoporosis and other bone-related disorders. These drugs contain varying alkyl chain lengths and moieties attached to two phosphonic acid groups. The testing of bisphosphonate drugs frequently involves derivatization to improve the reversed-phase retention and peak shape as well as the optical detectability of these highly acidic polar analytes. Here, the analysis of five bisphosphonate drugs using hydrophilic interaction liquid chromatography–mass spectrometry (HILIC-MS) is demonstrated without the need for derivatization. The optimized method using an Atlantis™ Premier BEH™ Z-HILIC Column achieves acceptable retention and peak shape for all five bisphosphonates as well as excellent column-to-column reproducibility.
Benefits
- Retention of underivatized bisphosphonate drugs using HILIC columns
- Improvements in peak shape with increased buffer concentration
- Excellent column-to-column reproducibility
Introduction
Bisphosphonates are a class of drugs first introduced in the 1970s and 1980s, with etidronic acid and clodronic acid showing some clinical success in the treatment of osteoporosis.1 The global market for bisphosphonate drugs was approximately 4.5 billion dollars in 2024 and is projected to grow to more than 6 billion dollars by 2033.2 These compounds are used in the treatment of bone-related disorders, including osteoporosis and Paget’s disease, and act by slowing down the resorption of bone by osteoclasts. These drugs all contain two phosphonic acid groups linked by an alkyl, alkylamine, or arylamine group. The first-generation bisphosphonate drug etidronic acid contains a methyl substituent, while newer drugs like risedronic acid and zoledronic acid contain a heterocyclic amine group, which improves their stability and functionality.
Many current methods for these analytes require derivatization with reagents such as trimethylsilyldiazomethane.3 Derivatizing the compounds converts the phosphonic acid moieties into phosphonate esters, which are neutral and less polar, allowing better retention in reversed-phase liquid chromatography (RPLC) while also improving the peak shape. Derivatization with o-phthalaldehyde or 9-fluorenylmethyl chloroformate has also been used to enable or improve the detection of bisphosphonates using ultraviolet or fluorescence detection.4 This methodology has been used extensively; however, recent advancements in column technology along with an improved understanding of alternative analysis techniques facilitate other avenues for the retention of these analytes without requiring derivatization. Using ion-exchange chromatography (IEX) or mixed-mode RP/IEX are viable alternatives as the retention is driven primarily or partially through ionic interactions. Alternatively, the use of HILIC allows these analytes to be retained by both ionic interactions and partitioning. HILIC is also highly compatible with mass spectrometry detection, which can provide greater sensitivity and specificity than other types of detection.
This application note describes the development of a HILIC method for five bisphosphonate drugs. Two different columns were tested, an ACQUITY™ Premier BEH Amide Column and an Atlantis Premier BEH Z-HILIC Column. Both columns employ MaxPeak™ High-Performance Surfaces (HPS) technology, which has been shown to improve peak areas and peak shapes for acidic analytes including those containing multiple phosphate groups.5–7 The BEH Amide Column utilizes 130 Å BEH particles bonded with amide groups and retains analytes primarily through partitioning.8 The BEH Z-HILIC Column employs zwitterionic sulfobetaine groups bonded on 95 Å BEH particles with a higher phase ratio, giving greater retention. The BEH Z-HILIC Column exhibits greater anion-exchange retention than the BEH Amide Column, making it particularly useful for ionized acids.8,9
Experimental
Sample Description
Stock solutions were created at 1 mg/mL in water using polypropylene scintillation vials and were stored at 4 °C. Aliquots of the stock solutions were removed and placed onto the system for analysis.
Method Conditions
|
LC system: |
ACQUITY Premier Binary Solvent Manager System with PDA Detector |
|
Detection: |
SIRs (ESI-) |
|
Column: |
Atlantis Premier BEH Z-HILIC, 2.1 x 50 mm, 1.7 µm (p/n: 186009978) ACQUITY Premier BEH Amide, 2.1 x 50 mm, 1.7 µm (p/n: 1860009504) |
|
Column temperature: |
30 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
2.0 µL |
|
Flow rate: |
0.5 mL/min |
|
Mobile phase A: |
80:20 Acetonitrile: Water (v/v) with 40 mM ammonium formate pH 3.0 (unless noted) |
|
Mobile phase B: |
50:50 Acetonitrile: Water (v/v) with 40 mM ammonium formate pH 3.0 (unless noted) |
|
Gradient conditions: |
Initial condition of 0% B. Linear gradient to 100% B in 6 minutes. Hold at 100% B for 1.15 minutes. Return to 0% B in 0.02 minutes and re-equilibrate for 1.16 minutes (unless noted) |
MS Conditions
|
MS system: |
Xevo™ TQ-S micro |
|
Detection: |
ESI- SIRs (see Figure 1) |
|
Capillary voltage (kV): |
3 |
|
Cone voltage (V): |
20 |
|
Desolvation temperature: |
350 °C |
|
Desolvation gas flow: |
650 L/hr |
|
Cone gas flow: |
0 L/hr |
Data Management
|
Chromatography software: |
MassLynx™ V4.1 |
Results and Discussion
To determine the best stationary phase for the analysis of the bisphosphonate compounds as shown in Figure 1, two columns were screened using generic HILIC screening conditions; a constant 10 mM ammonium formate pH 3.0 buffer was used with an aqueous gradient of 5–50% in 6 minutes. An ACQUITY Premier BEH Amide Column and an Atlantis Premier BEH Z-HILIC Column were tested and the resulting chromatograms for the two columns are shown in Figure 2.
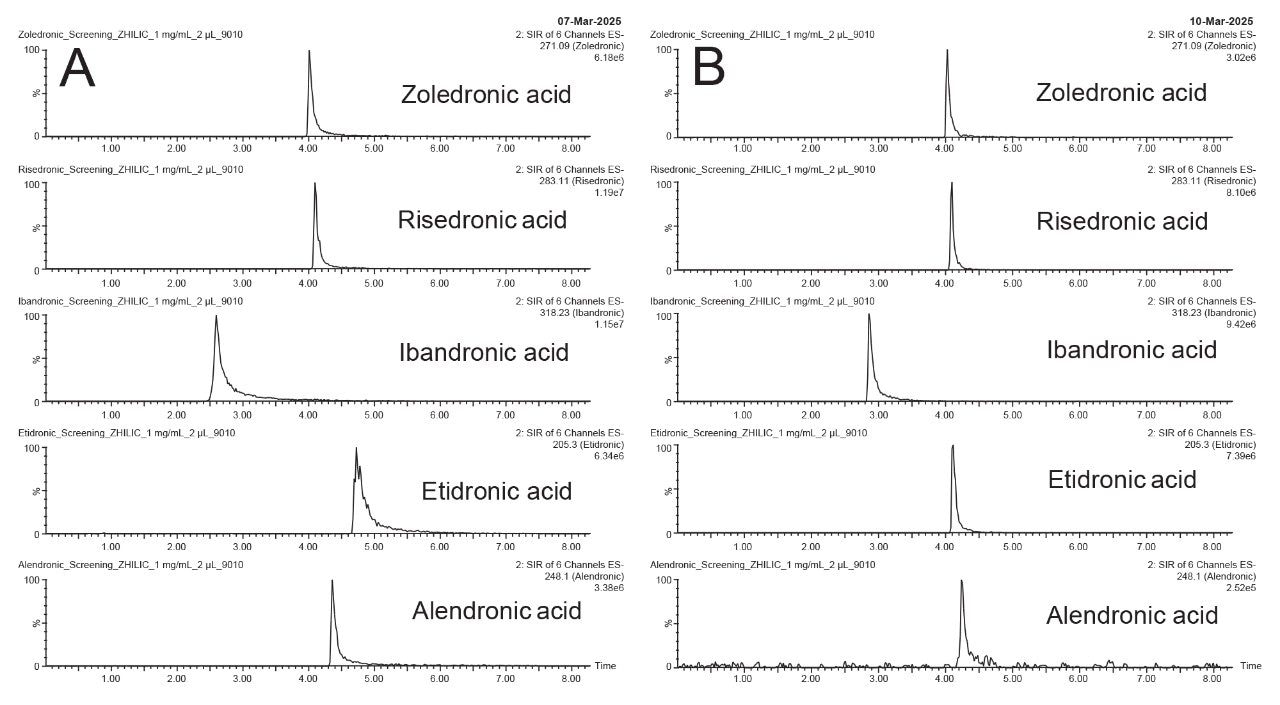
The elution order of the compounds is similar between the two columns, with ibandronic acid eluting first, followed by a grouping of alendronic, risedronic, and zoledronic acid. Etidronic acid is more retained on the BEH Z-HILIC Column due to the increased tendency of that stationary phase to retain via anion-exchange interactions compared to the BEH Amide stationary phase. Because it lacks an amine group, etidronic acid has a greater negative charge at pH 3 than the other four analytes. The BEH Z-HILIC Column gave slightly wider peaks and higher tailing factors for ibandronic acid and etidronic acid. Overall, both columns could be used for the analysis of bisphosphonates without derivatization; however, method optimization is needed to improve the peak shape obtained with the Atlantis Premier BEH Z-HILIC Column.
The first step to optimize this method is to try a higher buffer concentration. This not only leads to a thicker adsorbed aqueous layer, but also reduces the ionic interactions between the analytes and the stationary phase.9 Prior to testing an increased buffer concentration, a more focused separation using a 20–50% aqueous gradient was developed. This was needed to ensure that more concentrated buffers did not precipitate in the high acetonitrile content starting conditions. Once the new mobile phase system was determined, three buffer concentrations were tested: 10 mM, 20 mM, and 40 mM. The resulting chromatograms for the bisphosphonate compounds are shown in Figure 3.
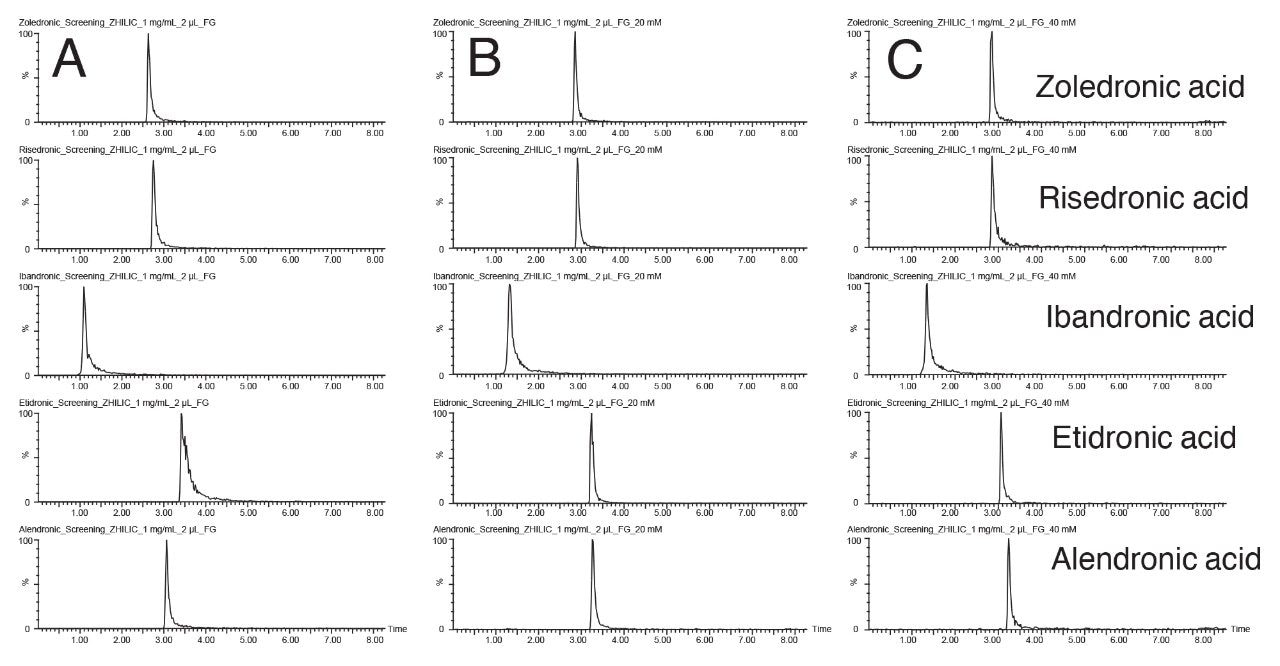
The use of higher buffer concentrations in the mobile phase had the expected impact on the peak shape of etidronic acid and ibandronic acid. The etidronic acid peak was noticeably sharper with 20 and 40 mM buffer compared to 10 mM, albeit eluting slightly earlier in the chromatogram. Ibandronic acid only exhibited a slight improvement in peak shape with the more concentrated buffers. With these improvements in peak shape, the Atlantis Premier BEH Z-HILIC Column is better suited for the analysis of these compounds compared to the original scouting conditions.
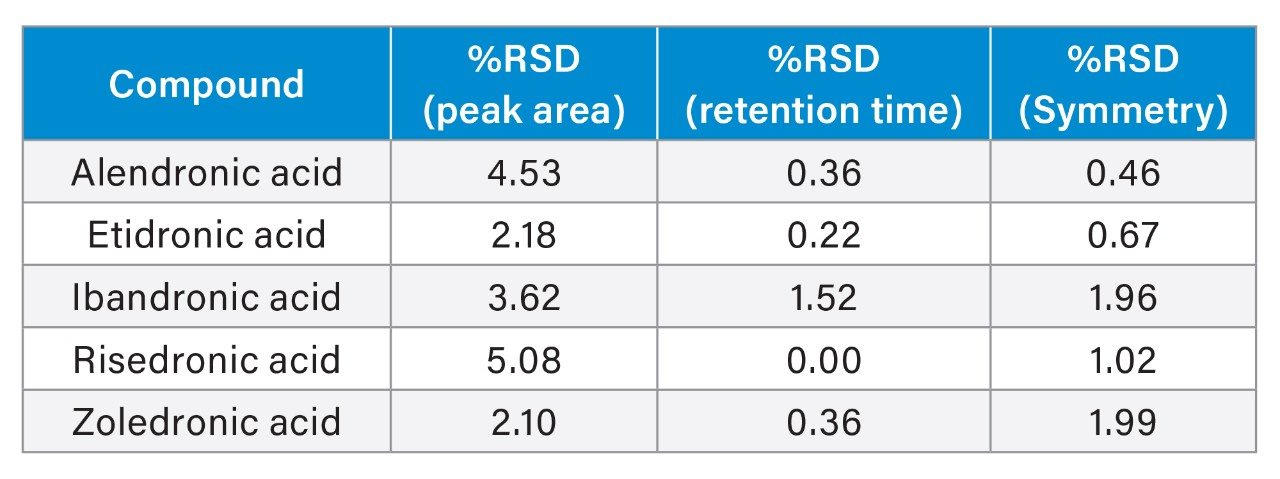
The optimized testing conditions using the Atlantis Premier BEH Z-HILIC Column with 40 mM ammonium formate pH 3.0 buffer were used to test column reproducibility. Three different columns were tested, packed at two different sites using two different batches of stationary phase. The resulting chromatograms are shown in Figure 4. Symmetry values for the peaks ranged between 1.14 and 1.44.
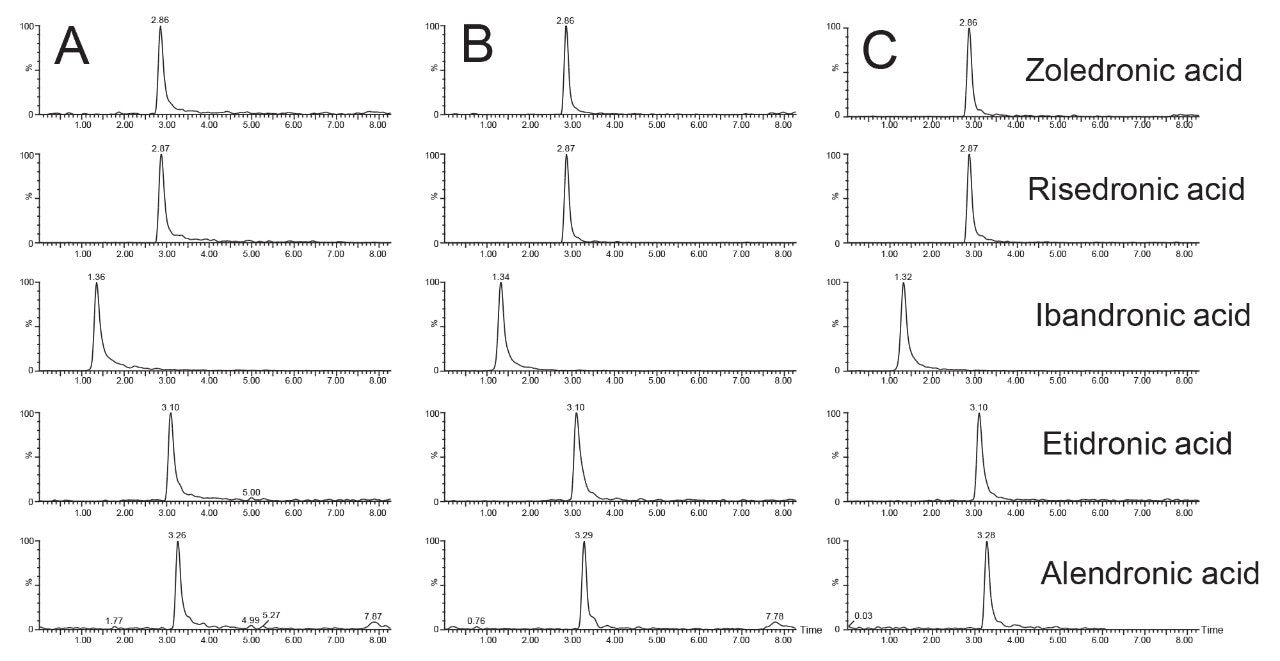
The three columns tested gave comparable results with no unexpected differences between the three. Peak area, retention time, and peak symmetry were monitored across all replicates on the columns and relative standard deviations (%RSD) were calculated for each (see Table 1). Peak symmetry was measured at 10% of peak height in the TargetLynx™ application of MassLynx. Good reproducibility was achieved using the optimized testing conditions. Slightly higher %RSD values were obtained for peak area, especially for risedronic acid. This may be due to slight sample degradation, or changes in MS sensitivity between injections or columns.
Using HILIC-MS for the analysis of bisphosphonate drugs eliminates the need for the extra sample preparation step of derivatization. As shown, good retention and peak shape and excellent column-to-column reproducibility can be achieved for these compounds with minimal method development or optimization required.
Conclusion
Bisphosphonate drugs have traditionally been analyzed using pre-analysis derivatization techniques to improve both their hydrophobicity and detection. While this is a viable option for the analysis of these compounds using RPLC, good retention and peak shape can be achieved using HILIC without the need for derivatization. In HILIC, polar analytes are retained through a combination of partitioning and ionic interactions. This makes HILIC ideal for retaining underivatized bisphosphonate drugs, which have a net negative charge above about pH 2.
Using the Atlantis Premier BEH Z-HILIC Column with an optimized gradient yields good retention and peak shape for underivatized bisphosphonate drugs as well as excellent column-to-column reproducibility. With this method, the derivatization step can be removed, improving not only throughput but also potentially reducing variability.
References
- Widler L, et. al. "Highly Potent Geminal Bisphosphonates. From Pamidronate Disodium (Aredia) to Zoledronic Acid (Zometa)". J. Med. Chem.. 45 (2002) 3721–3738.
- Bisphosphonate Drug Market Report Overview. https://www.businessresearchinsights.com/market-reports/bisphosphonate-drug-market-109707 Accessed 10 April 2025.
- Chen M, Liu K, Zhong D, Chen X. Trimethylsilyldiazomethane derivatization coupled with solid-phase extraction for the determination of alendronate in human plasma by LC-MS/MS. Anal. Bioanal. Chem. 402 (2012) 791–798.
- Manousi N, Tzanavaras PD, Zacharis CK. Determination of bisphosphonate active pharmaceutical ingredients in pharmaceuticals and biological materials: An updated review. J. Pharm. Biomed. Anal. 219 (2022) 114921.
- Delano M, Walter TH, Lauber M, Gilar M, Jung MC, Nguyen JM, Boissel C, Patel A, Bates-Harrison A, Wyndham K. Using Hybrid Organic-Inorganic Surface Technology to Mitigate Analyte Interactions with Metal Surfaces in UHPLC. Anal. Chem. 93 (2021) 5773–5781. doi: 10.1021/acs.analchem.0c05203.
- Walter TH, Alden BA, Belanger J, Berthelette K, Boissel C, DeLano M, Kizekai L, Nguyen JM, Shiner S. Modifying the Metal Surfaces in HPLC Systems and Columns to Prevent Analyte Adsorption and Other Deleterious Effects. LCGC Supplement (2022) 28–34.
- Smith K, Rainville P. Utilization of MaxPeak High Performance Surfaces and the Atlantis Premier BEH C18 AX Column to Increase Sensitivity of LC-MS Analysis. Waters Application Note 720006745.
- Gilar M, Berthelette K, Walter TH. Contribution of ionic interactions to stationary phase selectivity in hydrophilic interaction chromatography. J. Sep. Sci. 45 (2022) 3264–3275 doi: 10.1002/jssc.202200165.
- Walter TH, Alden BA, Berthelette K, Field JA, Lawrence NL, McLaughlin J, Patel AV. Characterization of a highly stable zwitterionic hydrophilic interaction chromatography stationary phase based on hybrid organic/inorganic particles. J. Sep. Sci. 45 (2022) 1389–1399. doi: 10.1002/jssc.202100859.
- Guo Y, Bhalodia N, Fattal B, Serris I. Evaluating the Adsorbed Water Layer on Polar Stationary Phases for Hydrophilic Interaction Chromatography (HILIC). Separations 6 (2019) 19. https://doi.org/10.3390/separations6020019.
720008862, June 2025