Advancing Analysis of Covalent High Molecular Weight Insulin With ACQUITY® Premier SEC 125 Å Columns
Abstract
Here we perform denaturing SEC analysis of human insulin with an ACQUITY Premier SEC 125 Å, 1.7 µm 4.6 x 150 mm Column demonstrating increased component resolution, shorter run times and more consistent recoveries of HMWPs compared to other commercially available SEC columns in addition to outstanding batch-to-batch and column-to-column reproducibility.
Benefits
- High resolution separation of insulin monomer and high molecular weight products (HMWPs) in less than 5 minutes
- MaxPeak™ High Performance Surfaces column hardware, innovative manufacturing processes and application specific batch testing helps ensure consistent, reliable insulin analysis
- High-throughput analysis significantly reduces mobile phase and sample consumption compared to analysis with traditional HPLC-based SEC columns as detailed in USP <121.1> and European Pharmacopoeia 9.0, 2017
Introduction
Insulin is a 5.8 kDa peptide hormone commonly formulated as a zinc-mediated noncovalent hexamer in pH neutral solutions that dissociates in vivo to produce the biologically active monomeric form involved in glucose regulation. Therefore, the efficacy of insulin drug products depends on the availability of insulin monomer and is negatively impacted by covalent aggregation.1 Insulin monomers are susceptible to partial protein unfolding and subsequent aggregation when exposed to certain environmental conditions commonly encountered during the production, storage and distribution of the drug substance and drug product.
Controlling the level of covalent high molecular weight products (HMWPs) is one of the critical quality attributes specified by the US and European Pharmacopeias (USP and EP) for insulin. The USP insulin monograph specifies a limit of NMT 1.0% HMWPs as determined by size-exclusion chromatography (SEC). Both the USP and EP monographs prescribe methods using a denaturing mobile phase comprising 65% 1 g/L arginine, 20% acetonitrile and 15% acetic acid. The acetonitrile and low pH of the mobile phase disrupts the zinc-mediated insulin hexamer and allows for quantification of monomeric insulin and covalent HMWPs.
Both the USP and EP monographs prescribe HPLC methods employing 300 mm columns with 7.8 mm inner diameters requiring run times of approximately 30 minutes, which consume large volumes of mobile phase and sample. Here we present data highlighting the performance benefits of using an ACQUITY Premier SEC 125 Å, 1.7 µm, 4.6 x 150 mm Column to achieve reproducible high-resolution separation of HMWPs from insulin monomer with a scaled version of the USP monograph method. The ACQUITY Premier SEC 125 Å, 1.7 µm Column enabled shorter run times along with reduced sample and mobile phase consumption compared to the USP monograph while providing superior resolution and HMWPs recovery compared to another commercially available sub-2 µm SEC column.
Experimental
Materials
All chemicals and reagents were of high purity and purchased from either Sigma-Aldrich or Fisher Scientific. Type 1 ultra-pure water from a Milli-Q IQ7000 (Millipore) was used for all aqueous solution and sample preparation.
Sample Description
Human insulin (Sigma-Aldrich p/n I2643) was reconstituted to 4 mg/mL in 0.01 N hydrochloric acid as prescribed by USP monograph and used as the insulin control sample in this application. Aliquots of the 4 mg/mL insulin control sample were incubated at 65 °C for 30, 60, or 90 minutes (thermally stressed) and then stored at 2–8°C prior to analysis. Insulin samples were analyzed without further dilution and within seven days of preparation.
ACQUITY Premier UPLC Conditions
|
Column: |
ACQUITY Premier SEC 125 Å, 1.7 µm, 4.6 x 150 mm (p/n: 186011350) |
|
Mobile phase: |
L-arginine (1.0 g/L) in H2O/glacial acetic acid/acetonitrile; 65/15/20 (v/v/v) |
|
Flow rate: |
0.4 mL/min |
|
Column temperature: |
25°C |
|
Sample temperature: |
10°C |
|
Injection volume: |
3.5 µL |
|
Detection wavelength: |
276 nm |
|
Needle purge: |
Milli-Q® Type 1 ultra-pure water |
|
Seal wash: |
10% methanol |
Results and Discussion
Our goal in this application was to explore the utility of a shorter (150 mm) ACQUITY Premier SEC 125 Å, 1.7 µm Column for the analysis of insulin under the same chromatographic conditions prescribed by USP <121.1>, though scaled for an ACQUITY Column. In addition, we aimed to characterize potentially significant batch-to-batch variations and compare performance against another commercially available sub-2 µm SEC column with similar pore diameter and particle size. We evaluated separation performance for analysis of the insulin control as well as insulin samples thermally stressed at 65 °C for different durations of time. Thermal stress increases the prevalence of partially unfolded insulin monomers in solution thereby accelerating formation of high molecular weight products (HMWPs). We monitored the initial increase in HMWPs in solution, which we anticipated to be pseudo-linear because of the negligible change in monomer concentration. 2
The resolution achieved with the ACQUITY Premier SEC 125 Å, 1.7 µm Column greatly exceeded the USP insulin monograph requirements despite using a column half the length prescribed by USP <121.1>. Run times for the ACQUITY method were 6x shorter (5 min vs. 30 min) and combined with the smaller 4.6 mm diameter column resulted in a 7x reduction in mobile phase consumption. Furthermore, nearly 30x less sample was required to achieve high resolution results (14 µg vs 400 µg). Figure 1 shows representative chromatograms using the industry standard Insulin HMWP 7.8 x 300 mm column (p/n: WAT201549) and the USP prescribed HPLC method compared with the method scaled for ACQUITY.
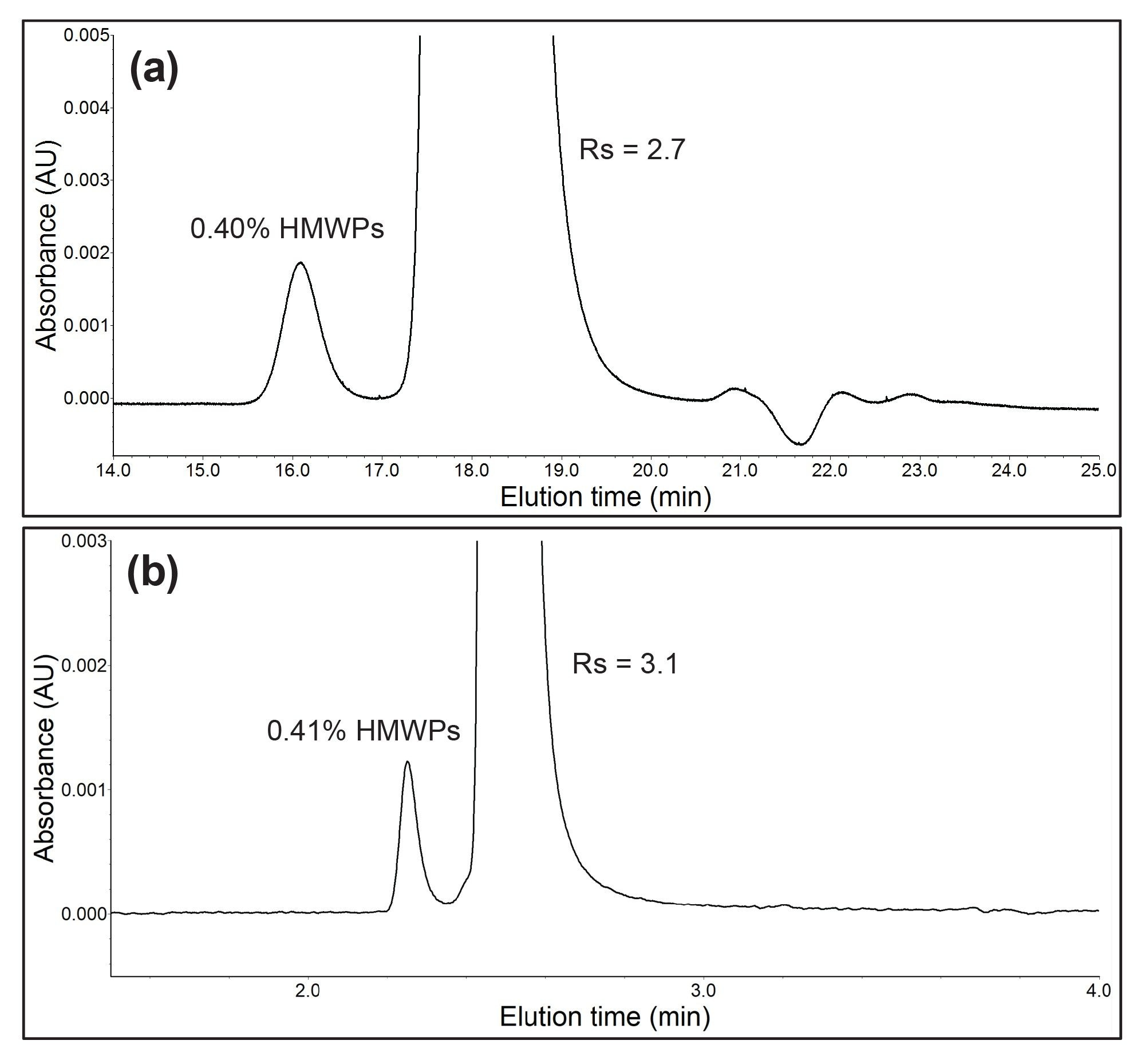
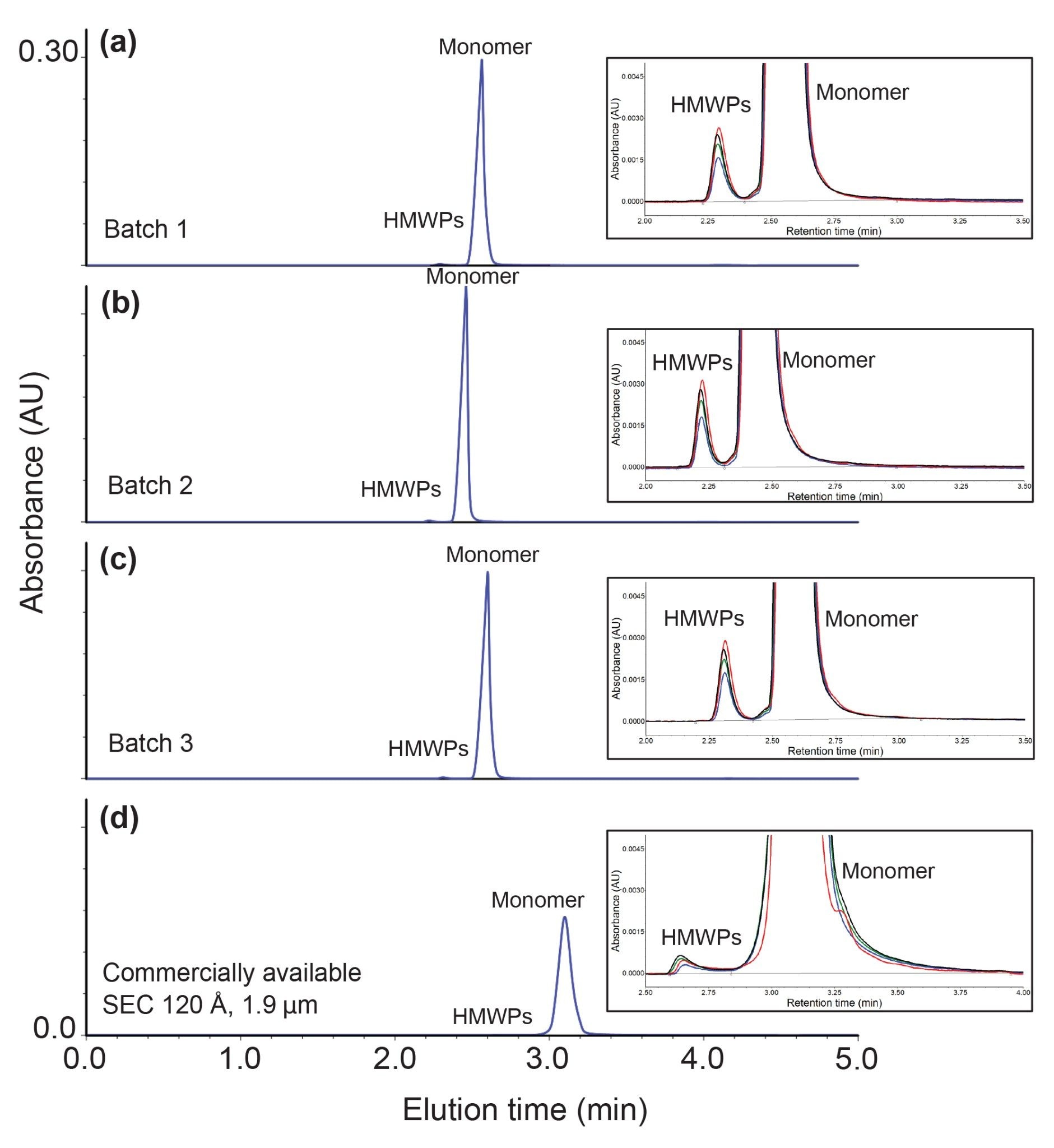
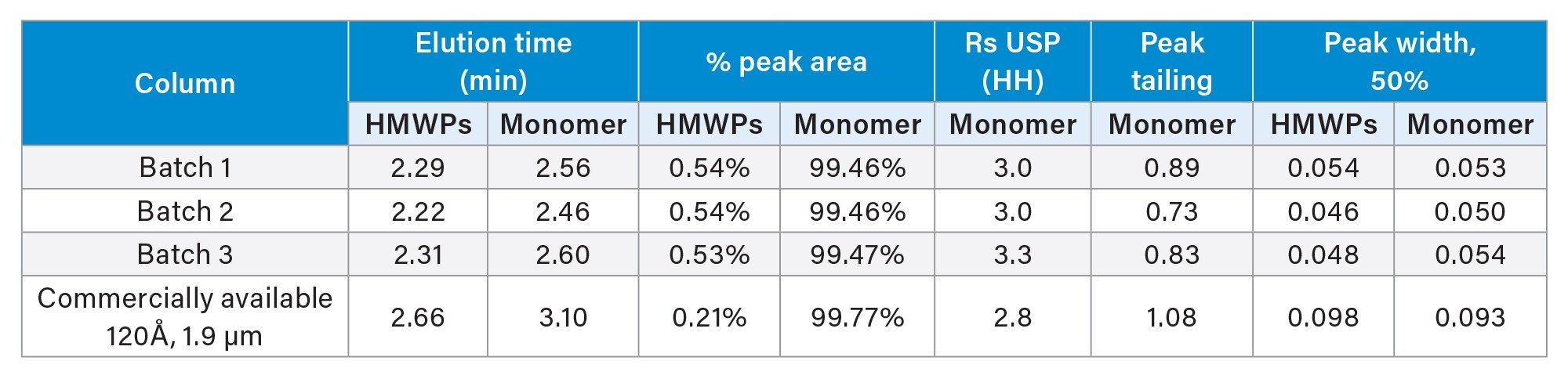
Figure 2 a–c shows the insulin chromatograms for three different synthesized batches of ACQUITY Premier SEC 125 Å, 1.7 µm Particles. Peak shape, peak intensity, and elution time were mostly unchanged across the different batches. The integration results summarized in Table 1 further demonstrate excellent reproducibility, with the resultant HMWPs % peak area nearly identical regardless of the batch tested.
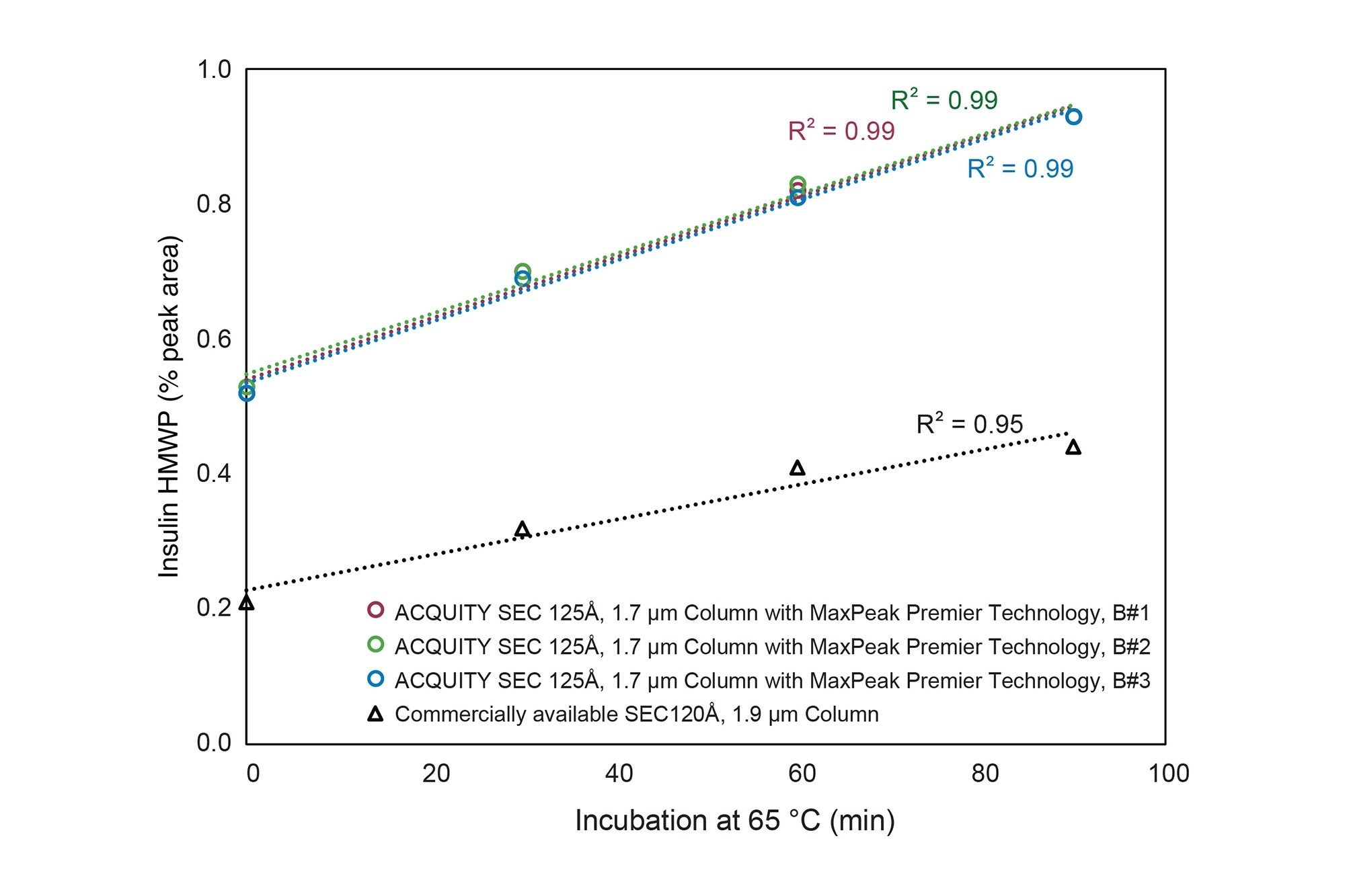
Figure 2 d shows the chromatogram for analysis of 4 mg/mL insulin with a commercially available 4.6 x 150 mm SEC 120 Å, 1.9 µm column. The ACQUITY Premier SEC 125 Å, 1.7 µm Column had narrower peaks and a greater than 2x increase in recovery of HMWPs compared to the commercially available column. Furthermore, the ACQUITY Premier SEC 125 Å, 1.7 µm Columns had consistently higher recoveries of HMWPs across the different thermal stress conditions (Figure 3). For the thermal stress conditions explored, we observed the expected linear increase in HMWPs when analyzing the samples with ACQUITY Premier SEC 125 Å, 1.7 µm Columns, but observed slight curvature in the data set generated with the commercially available comparative column (Figure 3). We hypothesize that secondary interactions may be causing some of the HMWPs to tail into the monomer peak during analysis with the comparative column, which would explain the lower observed HMWP peak areas.
Conclusion
We have demonstrated the utility of ACQUITY Premier SEC 125 Å, 1.7 µm 4.6 x 150 mm Columns for analysis of covalent insulin HMWPs. By using the shorter ACQUITY Column we obtained high resolution of the HMWPs and monomer peaks with higher throughput and significantly lower volumes of mobile phase and sample compared to the HPLC methods prescribed by USP and EP. The insulin-specific QC testing for every batch of ACQUITY Premier SEC 125 Å, 1.7 µm Particles ensures reproducibility over the life of a method. Finally, we demonstrated superior resolution and recovery of insulin HMWPs when compared to another commercially available sub-2 µm 120 Å SEC column.
References
- Anirban Das, M. S. (2022). Molecular Aspects of Insulin Aggregation and Various Therapeutic Interventions. ACS Bio&Med Chem, 205–221.
- Barnett, G. v., Drenski, M., Razinkov, V., Reed, W. F., & Roberts, C. J. (2016). Identifying protein aggregation mechanisms and quantifying aggregation rates from combined monomer depletion and continuous scattering. Analytical Biochemistry, 511, 80–91.
Featured Products
720008660, February 2025