Creating Greener HPLC Methods as Measured by the AMGS Metric: A Case Study of Improving USP Monograph Methods
Abstract
High Performance Liquid Chromatography (HPLC) is a common, well documented, and easily employed method for analyzing a wide variety of compounds. One area where HPLC plays a major role is in the pharmaceutical industry. Generic pharmaceutical companies and many quality control groups use HPLC systems and methods because they are often more accessible than newer technology, like Ultra-Performance Liquid Chromatography (UPLC™). In recent years, there has been a push to make analytical chemistry techniques, like HPLC, more sustainable. These “green” initiatives focus on using fewer toxic reagents, and reducing waste and energy consumption, while still achieving desirable scientific results.
This application note examines the USP monograph procedure for the analysis of rivaroxaban and related impurities. A new method was generated in-house with green principles in mind. All methods were evaluated and scored using the Analytical Method Greenness Score (AMGS) method and their scores listed. The new method generated in-house had a greener score than the USP monograph conditions.
Benefits
- Development of a new, greener HPLC method for rivaroxaban and impurities, as outlined in USP monograph
- Implementation of the AMGS metric to evaluate the sustainability of analytical methods
- Comparison of AMGS scores for the two methods
Introduction
The use of High Performance Liquid Chromatography (HPLC) is well documented and very well known in certain scientific communities. Several industries rely heavily on Liquid Chromatography (LC) to achieve their goals, whether that be releasing a formulated drug product, monitoring patient health, or developing a new packaging material. HPLC is prevalent in pharmaceutical, food, environmental, materials science, academic, and bioanalytical workflows to varying degrees. The pharmaceutical industry is one of the largest that takes advantage of this proven technology, especially for quality control. The accessibility of the technology, coupled with many years of experience, make it ideally suited for this industry. However, while used to great effect, HPLC still has several drawbacks, including the lower overall throughput compared to newer technologies like UPLC, and significant volumes of waste being generated. The latter issue is of particular interest lately, as “green” initiatives are gaining momentum around the world. Making HPLC methods more sustainable can not only decrease the negative impacts on the environment and human health but can also reduce costs.
Analytical techniques like HPLC can be assessed for “greenness” using several scoring metrics. One such methodology is called the Analytical Method Greenness Score (AMGS), which was proposed in 2019 by scientists from several pharmaceutical companies.1 This methodology takes into consideration the instrument energy usage, run time, and number of injections to first calculate an Instrument Energy Score. Next, a Solvent Energy Score is calculated, factoring in total solvent volume, solvent density, and solvent energy demand contribution. This score is a measure of the energy used to produce and dispose of the solvents. Lastly, a Solvent EHS (environmental, health, and safety) score is calculated by including solvent volume, solvent density, and the EHS average, which is determined by an unknown method by the calculator. These three scores are then combined to create a greenness score, where the lower the number, the greener the method. Additionally, the AMGS methodology indicates the percentage of contribution to the greenness score from each individual score to allow further improvements to be made to individual aspects of the method, such as moving to an analytical system that requires less energy to operate. The calculator is available as a free online tool on the ACS Green Chemistry Institute Website.2
This application note focuses on improving the USP monograph method for rivaroxaban and impurities. Rivaroxaban is sold under the trade name Xarelto and is used to treat and prevent blood clots, specifically deep vein thrombosis and pulmonary emboli. It is also used to prevent blood clots after major operations, such as knee or hip surgeries. This compound has validated USP monograph methods for both assay and impurity analysis that use both potassium phosphate and sodium hexane sulfonate. Both additives are used in the mobile phase and, therefore, are considered waste products of the analysis. Additionally, sodium hexane sulfonate, which acts as an ion-pairing agent, can be particularly problematic in HPLC systems, as the removal of the additive can take a very long time if not done properly, as it requires flushing the system to allow the system to de-passivate. A new method was developed to separate rivaroxaban and its four impurities as listed in the USP monograph. For both the USP monograph conditions, as well as the newly developed method conditions, AMGS methodologies were applied, and scores were generated using the online tool.2
Experimental
Method Conditions
Sample Description
Stock solutions of rivaroxaban and related compounds were made at 1 mg/mL each using 40:60 v/v water:acetonitrile as the diluent. Stock solutions were diluted to 0.5 mg/mL (rivaroxaban) and 10 µg/mL each (related compounds B, D, G, J).
LC Conditions
|
LC system: |
Alliance™ e2695 HPLC System with 2489 UV/Vis Detector |
|
Detection: |
UV at 250 nm |
|
Columns: |
XSelect™ Premier HSS T3, 4.6 X 100 mm, 3.5 µm Column (p/n: 186010935) |
|
Column temperature: |
30 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
3.0 µL |
|
Flow rate: |
1.0 mL/min |
|
Mobile phase A: |
Milli-Q Water |
|
Mobile phase B: |
Ethanol |
|
Gradient conditions: |
Initial conditions of 5% B. Linear gradient of 5–95% B in 16.43 minutes. Hold at 95% B for 2.76 minutes. Return to 5% B and hold for 5.5 minutes. Total cycle time: 25 minutes. |
Data Management
|
Chromatography software: |
Empower™ 3 Feature Release 4 |
Results and Discussion
Method Development of Rivaroxaban and Impurities
The USP monograph method for rivaroxaban and impurities specifies the use of potassium phosphate and sodium hexane sulfonate in the mobile phase, along with both methanol and acetonitrile strong solvents.3 While this method may be suitable for the analysis of the active ingredient rivaroxaban, as well as its synthesis impurities, the described mobile phase composition is less than ideal. Not only does the method require a multi-step process to create the mobile phase, but the sodium hexane sulfonate, being an ion-pairing agent, requires a lengthy conditioning time on a new system. Additionally, because the ion-pairing agent can contaminate a system due to passivation with metal surfaces, removing the additive can be problematic. This essentially requires a dedicated system for the assay, as swapping between mobile phase systems would be difficult. On top of the above-described issues, the USP monograph method is far from sustainable due to the high flow rate and lengthy analysis time per injection. Having a more sustainable method with mobile phases that are easier to make would not only decrease its environmental impact, but also reduce the potential for mistakes during mobile phase creation. To that end, a new method for separating rivaroxaban and the four impurities specified in the USP monograph was developed.
A simple screening gradient (5–95% organic) was chosen to first scout the conditions needed to separate the five analytes. While not the obvious first choice for a method, ethanol was selected as the strong solvent. Ethanol is a more sustainable solvent choice, as it is considered a renewable solvent, because it can be created via fermentation of plant matter as opposed to petrochemical processes. An XSelect Premier HSS T3 Column was selected, as the MaxPeak™ Premier Hardware employs MaxPeak High Performance Surfaces (HPS) Technology, which mitigates non-specific adsorption between analytes and metal components of the LC system and column.4 If the analytes are subject to this non-specific adsorption effect, the use of a MaxPeak Premier Column will eliminate that interaction, providing more accurate and reproducible results. Reducing the risk in the development of new methods is critical to finding an answer sooner and avoiding future problems. Figure 1 shows a stacked plot of three replicate injections of the rivaroxaban and impurities sample on the XSelect Premier HSS T3 Column using a linear 5–95% ethanol gradient at a slope of 3% ethanol per column volume. No mobile phase additives were used for this method.
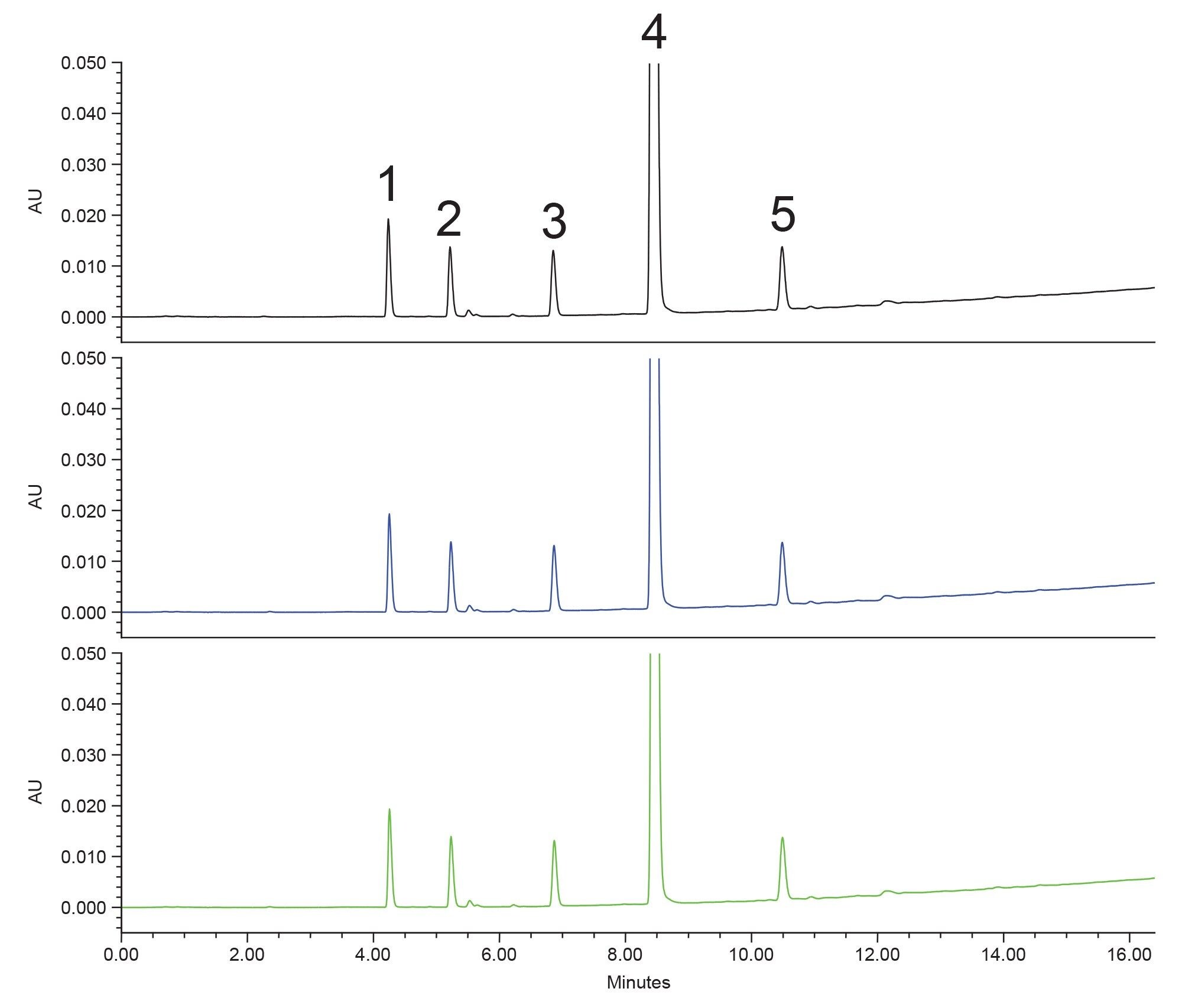
This method not only meets all assay criteria, excluding sensitivity which was not tested, but is also faster in terms of analysis time. The USP monograph method calls for a 37-minute gradient, not including re-equilibration time. The newly developed method has a 25-minute cycle time, improving throughput overall. This translates directly into productivity improvements, while also reducing the cost of analysis and using less mobile phase. Additionally, since the new mobile phase system only uses water and ethanol, the time to prepare the mobile phase is significantly reduced while making the process simpler to follow. This new method improves throughput and decreases solvent usage by approximately 63% compared to the compendial procedure.
Green scores can be calculated for this new method and the original USP monograph conditions. However, for these calculations, a few assumptions must be considered, which apply because of the intended use of the method. Since this method could, in theory, replace the USP monograph method, some additional factors must be considered. For instance, replicate injections of a sample are needed to meet certain USP criteria. Specifically, the USP monograph calls out a %RSD of no more than (NMT) 5% for the peak area of rivaroxaban. Based on USP General Chapter <621>, for that requirement, a total of 6 replicates are needed. Additionally, the monograph method calls for a total of three samples, including a standard solution and a sensitivity solution. Therefore, for this assay, a total of 8 injections are needed to complete the testing, with three different samples being prepared. This number will be applied for all green scoring calculations where applicable, along with the appropriate mobile phase and gradient conditions.
Green Scoring Using the AMGS Metric
When using the AMGS online calculator, the first parameter to input is the technique. The choices provided are fairly broad and cover the typical analytical techniques, including Prep LC, SFC, LC-MS, and NPLC. For both the USP monograph and the newly developed method, the technique is HPLC. The calculator applies an appropriate rate of energy consumption to calculate the Instrument Energy Score. The next input is the number of analytes of interest and the number of injections for a full analysis. These values are the same for both the USP monograph conditions and the newly developed method. The number of analytes of interest are five, which includes rivaroxaban and the four impurities, and the total number of injections needed to meet the monograph requirements is eight.
The next part of the calculator handles the instrument conditions, including solvent composition, run time, flow rate, and gradient conditions. This is where the two methods differ greatly. The differences discussed previously were entered into the calculator. Additionally, the USP monograph conditions list a 37-minute method but do not include column re-equilibration time. For this calculation, a 40-minute run time was input to account for re-equilibration between injections. One downside of this calculator is that it does not take into consideration any mobile phase additives for either the Solvent Energy Score or the Solvent EHS Score. The USP monograph method calls for sodium hexane sulfonate which has different safety considerations than formic acid, for instance. Mobile phase composition in terms of organic percentage does need to be specified, however.
Next, the calculator requires inputting information about samples and stock solutions. While typically a USP monograph is for the analysis of live samples, in this case, only stock solutions are needed. As such, values for a live sample are not relevant. However, the calculator still requires a value to be entered for sample diluent, sample volume, and number of sample preps for live samples. For this application note, as there are no live samples being tested, values of 0 were added for both, with a solvent composition of 100% water. The calculator asks for sample prep volumes and the number of stock solutions, along with the diluent being used as a mixture of water and acetonitrile. For this application note, stock standard prep volume was input at 5 mL with a total of five stock solutions created, one for rivaroxaban and each of its impurities. A diluent of acetonitrile and water (40:60) was used.
Next, the values for working standards are needed. For this application note, while not examined, the USP monograph does call for a sample to be analyzed. Given that the sample is not needed in replicate, and is only injected once, a volume of 1 mL and one prep was input into the calculator, with a diluent of 40:60 acetonitrile:water. This finishes the needed information for the working standard preparation. System suitability samples are next recorded. Only one prep of the sample is needed, and the volume needed is 1 mL to allow for enough replicates to be injected. The diluent of 40:60 acetonitrile:water was used here as well. The last piece of information needed is sensitivity solution preparation. For this work, while not shown, a sensitivity sample would be needed. With only one injection required, the number of preps was input as one with a volume of 1 mL. The standard sample diluent listed above was also applied for this standard. Once all that information is entered, the total scores can be calculated. Table 1 shows the AMGS scores for both the USP monograph conditions as well as the newly developed method.
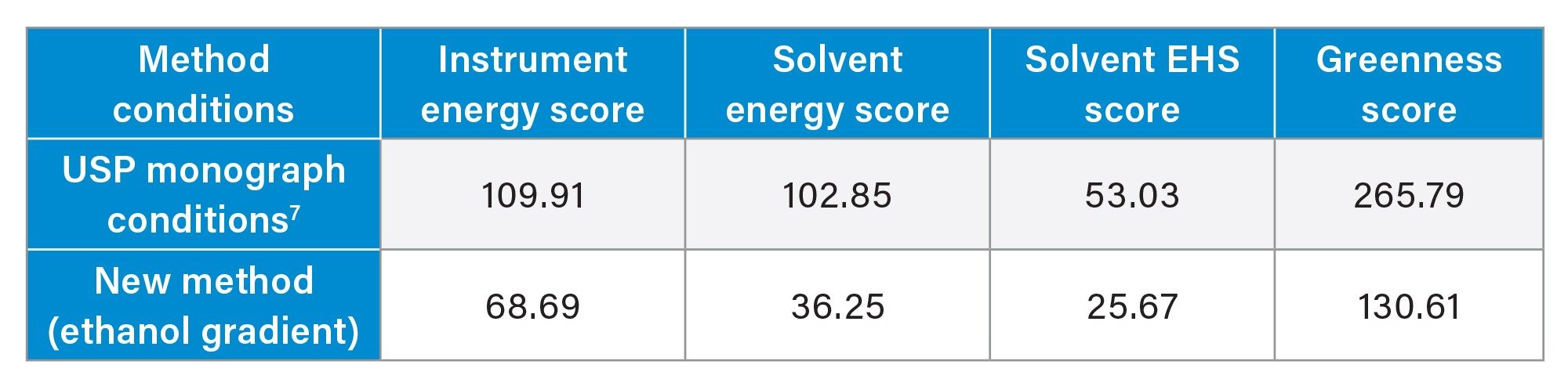
As the scores clearly show, the newly developed method is more sustainable than the USP monograph conditions. Not only is the greenness score reduced by ~50% compared to the USP monograph conditions, but notable differences are seen in the individual scores. Most notable is the Solvent Energy Score, which is considerably lower with the new method compared to the USP monograph conditions. This is likely due to the fact that the USP monograph conditions use multiple organic solvents, none of which are considered “bio-based”, while the new method uses only ethanol. It should be noted that since the same number of analytes and samples are needed, and the same instrument is being used, the difference in Instrument Energy Score between the two conditions is directly related to run time.
AMGS scores are a suitable way to measure the greenness of a method but should only be a deciding factor once a method achieves the desired scientific results. AMGS scores can be a good deciding factor between two equally suitable methods that both achieve the desired scientific result. To that end, the newly developed method would be preferred, as it shows separation of the impurities listed in the USP monograph, while having a lower AMGS value than the original USP monograph conditions.
Conclusion
Sustainability efforts are coming to the foreground for many workflows, including but not limited to, synthetic chemistry and analytical chemistry. There are a variety of ways to improve the “greenness” of an analytical method, including using bio-based solvents, reducing waste generation, and using instrumentation with lower power consumption. Of course, a balance must be struck between the sustainability of a method and its use to generate high-quality, fit-for-purpose data. Many older HPLC methods use mobile phase additives which are not the most sustainable. Additionally, they often use older column technology and instrumentation that generate a lot of waste, further reducing their “greenness”. By developing a new method which still meets the needs of the analysis, but keeping green principles in mind, more sustainable methods can be generated. This application note focuses on the development of a new method to separate rivaroxaban and its impurities listed in the USP monograph. Comparisons of AMGS scores, a measure of sustainability for analytical methods, was made between the new method and the USP monograph conditions. By keeping green principles in mind, more sustainable methods can be developed which not only reduce operational cost, but also decrease the environmental impact of the laboratory.
References
- Hicks M, Farrell W, Aurigemma C, Lehmann L, Weisel L, Nadeau K, Lee H, Moraff C, Wong M, Huang Y, Ferguson P. Making the Move Towards Modernized Greener Separations: Introduction of the Analytical Method Greenness Score (AMGS) calculator. Green Chem. 21 (2019) 1816
- ACS Green Chemistry Institute Pharmaceutical Roundtable Website. Accessed 14-Feb-2024.
- USP Monograph Rivaroxaban. https://online.uspnf.com/uspnf/document/1_GUID-80A96549-FD91-4801-8878-23D22A9616D5_2_en-US?source=Search%20Results&highlight=Rivaroxaban Accessed 8-Feb-2024.
- Lauber M, Walter TH, Gilar M, DeLano M, Boissel C, Smith K, Birdsall R, Rainville P, Belanger J, and Wyndham K. Waters Corp. White Paper 720006930, 2020.
Featured Products
720008281, March 2024