
This application note describes how the AstraZeneca QC department in Macclesfield has successfully transferred and run all registered QC methods on the Waters ACQUITY UPLC H-Class System with three high throughput products that were successfully developed and validated for UPLC.
In this application note, we describe how the AstraZeneca QC department in Macclesfield (a global center for developing new technologies for QC) has successfully transferred and run all registered QC methods on the Waters ACQUITY UPLC H-Class System with three high throughput products that were successfully developed and validated for UPLC. In efforts to update and modernize their labs, it was critical for Astra Zeneca to ensure new technology would be efficient, easy to adopt, and cost effective. AstraZeneca updated their LC platforms by implementing Waters UPLC in their pharmaceutical development department with the intention of developing all new products on this platform. While future-proofing the QC department to receive newer UPLC methods, it was critical to retain the ability to faithfully and robustly run legacy chromatography methods. The technology of choice was the ACQUITY UPLC H-Class System, which has now been deployed throughout the AstraZeneca QC department based at Macclesfield, UK.
Within this body of work, we will give an example of a high profile compound ‘B’ legacy HPLC method transferred from an Agilent 1100 to a Waters ACQUITY UPLC H-Class System along with the newly developed UPLC method validated on the same instrument.
The new UPLC method for compound B degradant products was created using the ACQUITY UPLC Columns Calculator, to simplify transfer and scale HPLC methodology quickly to UPLC conditions with equivalent performance (with significantly reduced runtimes and solvent savings) ensuring it satisfied the system suitability criteria stated in the legacy HPLC method.
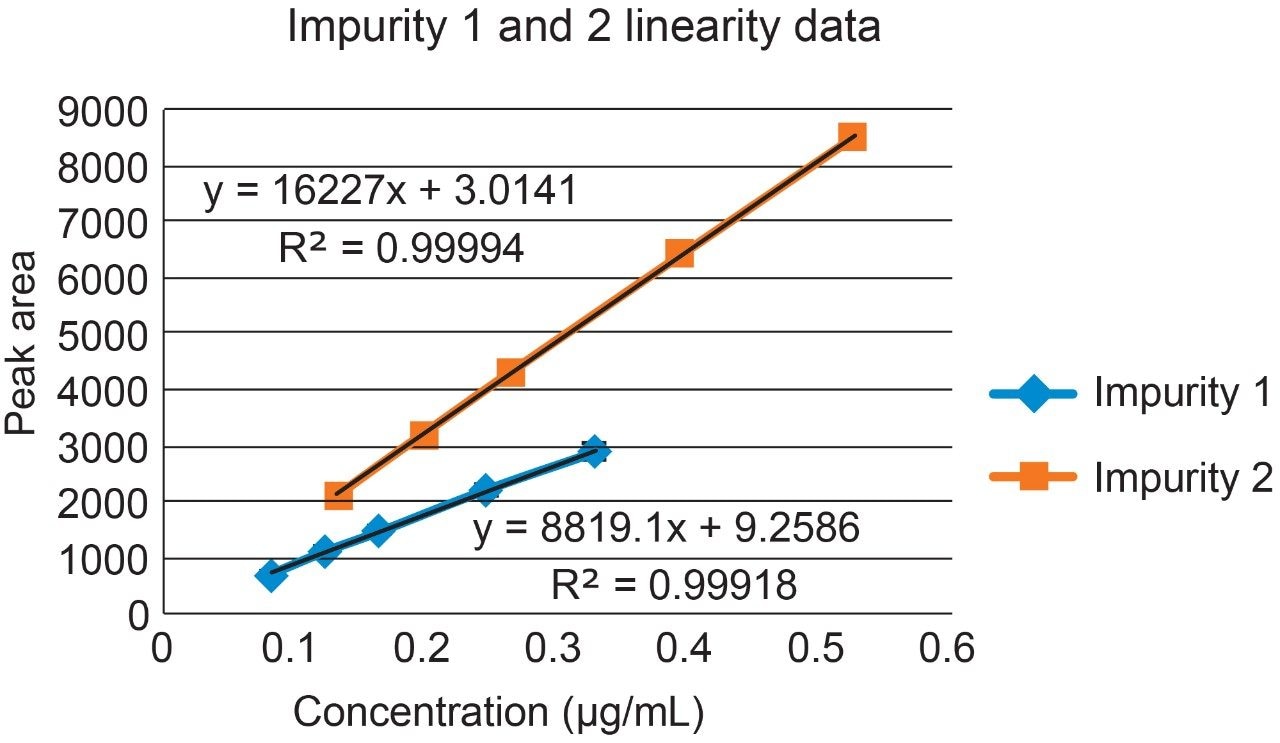
Impurities 1 and 2 of compound ‘B’ were validated over a range of 50% to 200% of their respective specification limits in the presence of the main compound ‘B’.
|
Column: |
C8 4.6 mm × 250 mm, 5 μm |
|
Flow rate: |
1.3 mL/min |
|
Injection volume: |
50 μL |
|
Run time: |
30 min |
|
Detection: |
UV |
|
Column: |
Waters ACQUITY UPLC BEH 2.1 mm × 100 mm, 1.7 μm Column |
|
Flow rate: |
0.3 mL/min |
|
Injection volume: |
4.2 μL |
|
Run time: |
6.86 min |
Empower 2 CDS (Chromatography Data System) Software
For the ACQUITY UPLC H-Class to be a successful forward facing platform for the quality control environment, it must first be able to faithfully and robustly reproduce the chromatography generated on the laboratory’s existing HPLC platform. Figure 1 shows the comparison of Compound B’s system suitability sample (SST) run on the Agilent 1100 (top), the Waters ACQUITY UPLC H-Class System in HPLC mode (middle), and the ACQUITY UPLC H-Class System again using the newly developed UPLC method (bottom).
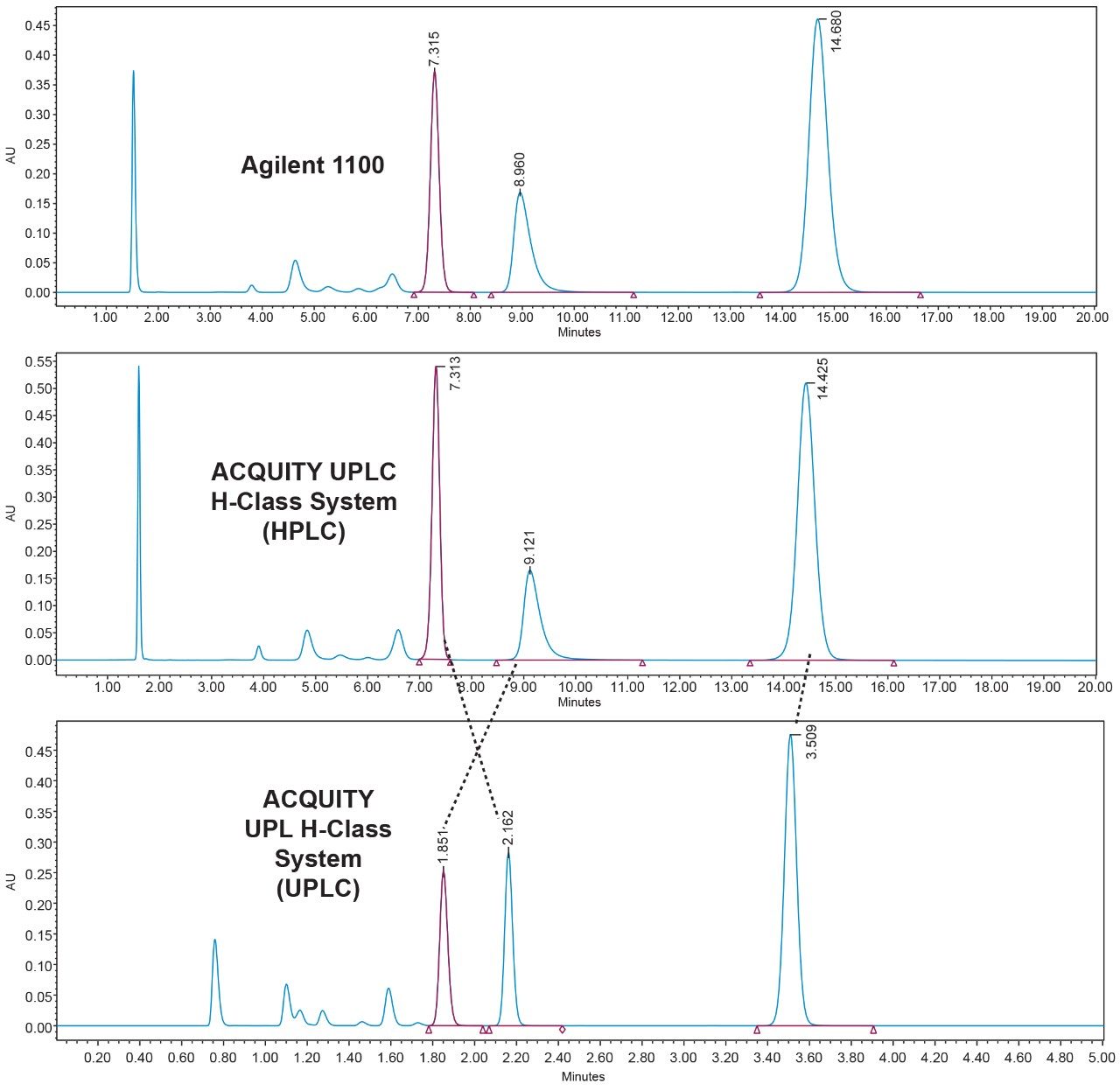
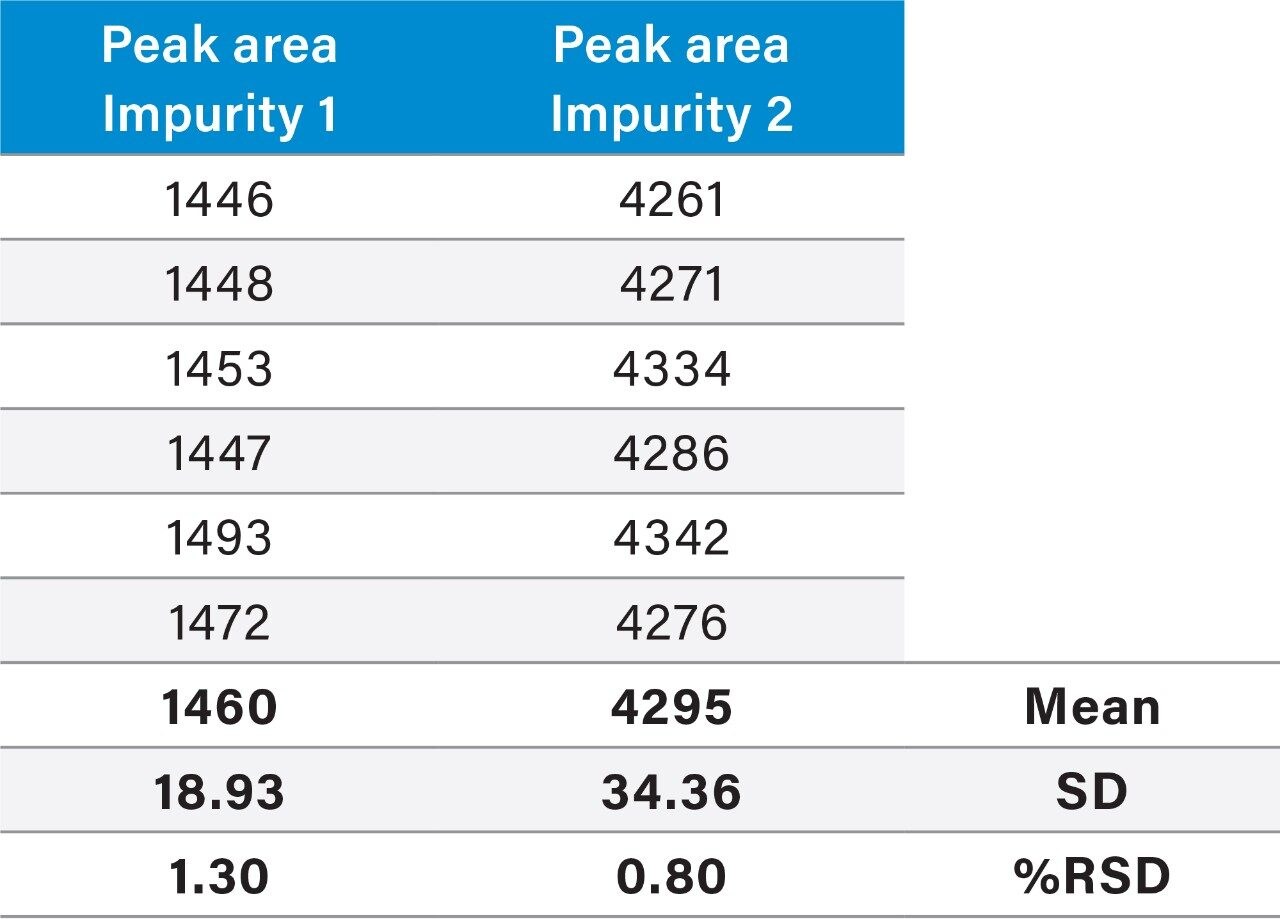
The ACQUITY UPLC H-Class System has reliably replicated the chromatography from the Agilent 1100 and reproduced the relative retention times (RRT’s) of impurities 1 and 2 with respect to the main peak, as shown in Table 1. In HPLC mode, the ACQUITY UPLC H-Class System also consistently reproduced the peak areas of Compound B and its related impurities compared to those obtained on the legacy LC system, as shown in Table 2.
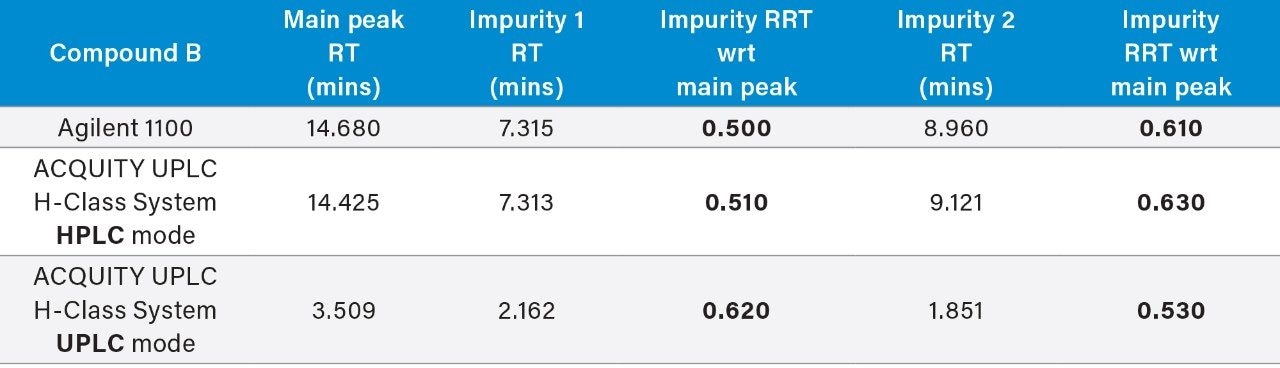
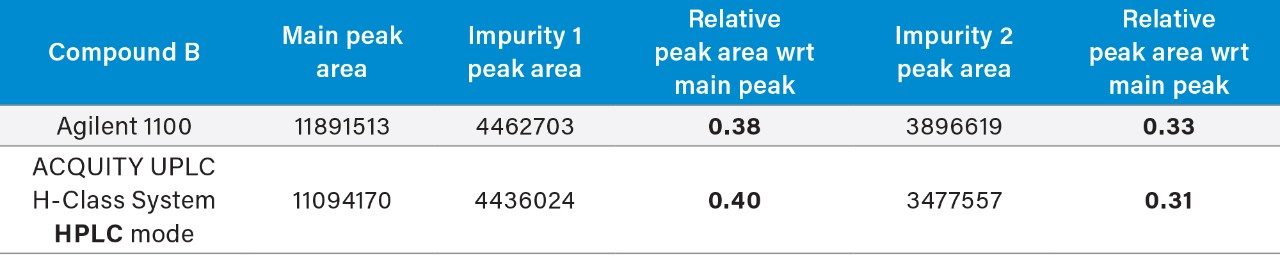
The UPLC method had a runtime of under seven minutes compared to the legacy method runtime of 30 minutes. There is also a marked improvement in peak symmetry. Impurities 1 and 2 have switched elution order, although this has not compromised system suitability criteria as shown in Figure 1.
Once the newly developed UPLC method for Compound B degradants had satisfied system suitability criteria, the method was subject to a partial validation based on ICH Guidelines Q21 covering linearity, recovery, repeatability, and limits of detection and quantitation (LOD and LOQ respectively).
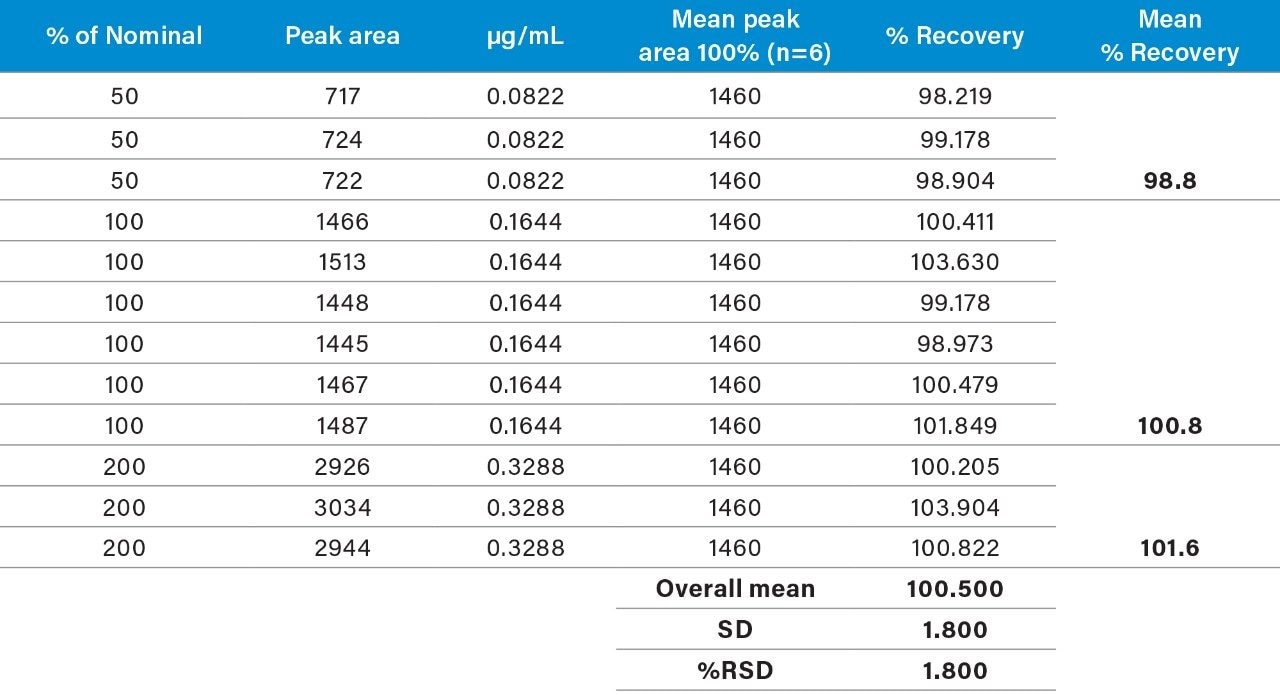
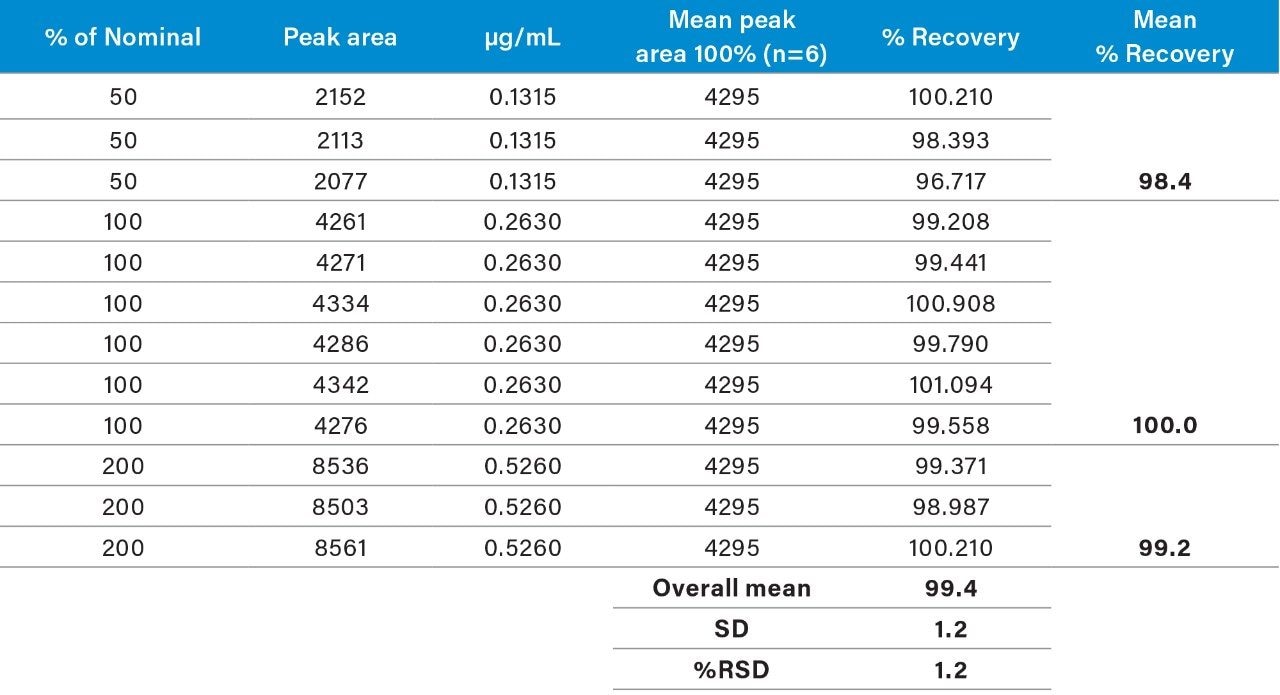
The range of the the validation covered 50% to 200% of the impurities respective specification limits (this exceeds the ICH Guideline’s suggestion of 70% to 130% for added assurance of method robustness).2
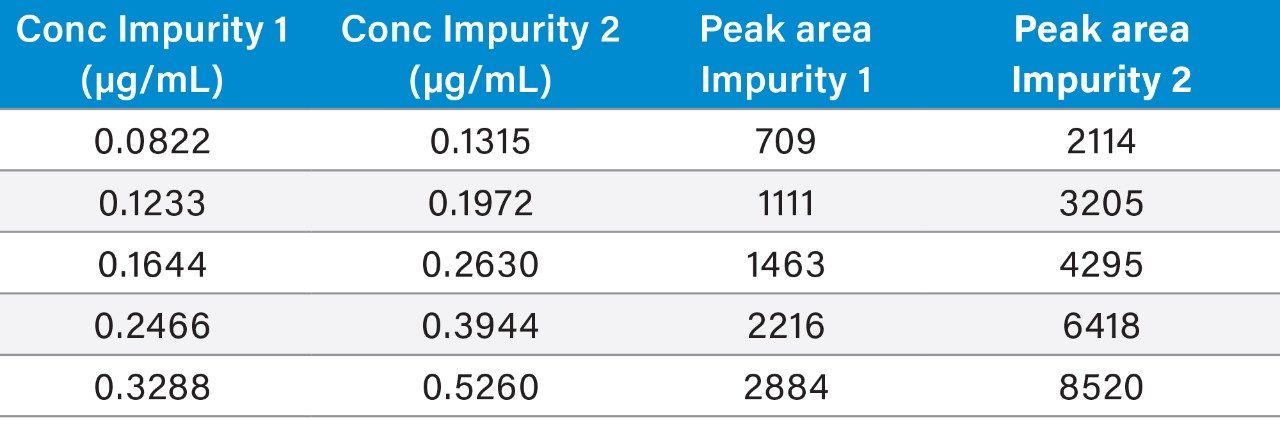
Table 3 summarizes the validation data obtained.
Raw linearity data is presented in Figure 2 and Table 3, method precision data in Table 4, and impurity 1 and 2 recovery raw data is presented in Tables 5 and 6 respectively.
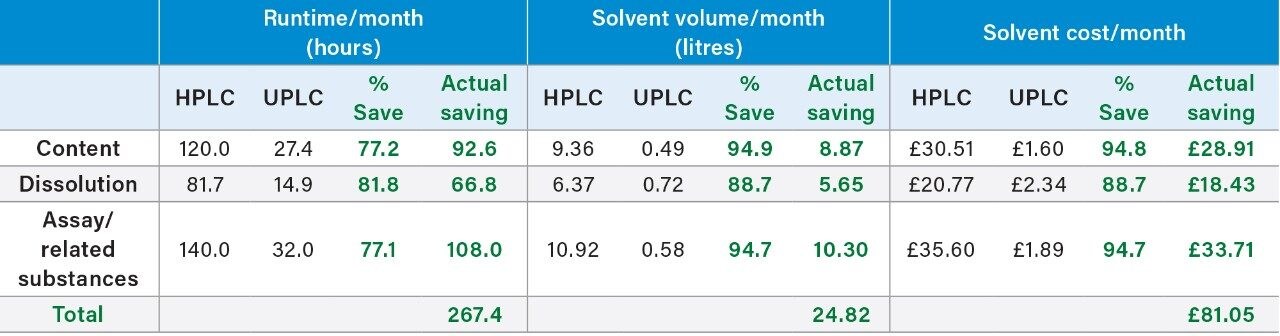
The UPLC data detailed for Compound ‘B’ shows a time savings of between 77.1% to 81.8% equating to over 267 hours per month with solvent savings between 88.7% to 94.8% per month. This not only impacts solvents costs associated with purchase and disposal, but also reduces the need for large storage volume impacting space savings and health and safety.
The Waters ACQUITY UPLC H-Class System’s success in transitioning legacy methods within the Quality Control environment of AstraZeneca exemplifies the instrument’s ability to offer a seamless alternative to existing HPLC platforms while uniquely offering the option of true UPLC Technology when desired.
AstraZeneca have successfully run all registered QC methods on the ACQUITY UPLC H-Class System with three high throughput products transferred and validated successfully using UPLC Technology.
The success of the Waters ACQUITY UPLC H-Class System in the Quality Control department of AstraZeneca Macclesfield has led to a wider adoption globally of the ACQUITY UPLC H-Class System by AstraZeneca.
720006615, July 2019