
This application note presents the transfer of an HPLC separation of 16 cannabinoids to UPLC resulting in a greater than 2-fold increase in productivity while maintaining the linearity, selectivity, and suitability observed in the HPLC separation.
The ACQUITY UPLC H-Class System is a highly reliable and robust instrument that works in combination with the CORTECS Column chemistry to provide high throughput analysis of complex cannabinoid mixtures, such as cannabis flower and concentrate.
As the legalization of cannabis for both medicinal and recreational use continues to advance, the need for simple, reliable analytical methods for the analysis of these products is desired by many parties (producers, regulators, and consumers).
This application note will present the modification of a simple isocratic HPLC separation of 16 cannabinoids1 to a separation under UPLC conditions. Conversion of the method to UPLC operation provides a greater than 2 fold increase in testing productivity while maintaining the linearity, selectivity, and suitability observed in the HPLC separation.
|
LC system: |
ACQUITY UPLC H-Class |
|
Analytical column: |
CORTECS UPLC Shield RP18, 90 Å, 1.6 μm, 2.1 × 100 mm (p/n: 186008694) |
|
Analytical flow rate: |
0.7 mL/min |
|
Mobile phase A: |
Water with 0.1% TFA |
|
Mobile phase B: |
Acetonitrile |
|
Isocratic: |
41:59 mobile phase A/mobile phase B |
|
Oven temp.: |
35 °C |
|
Detector: |
ACQUITY UPLC PDA |
|
Detection wavelength: |
228 nm at 4.8 nm resolution |
|
Injection volume: |
0.7 μL for 1.0 mg/mL reference standard preparations, sample solutions scaled appropriately |
|
Software: |
Empower 3 CDS |
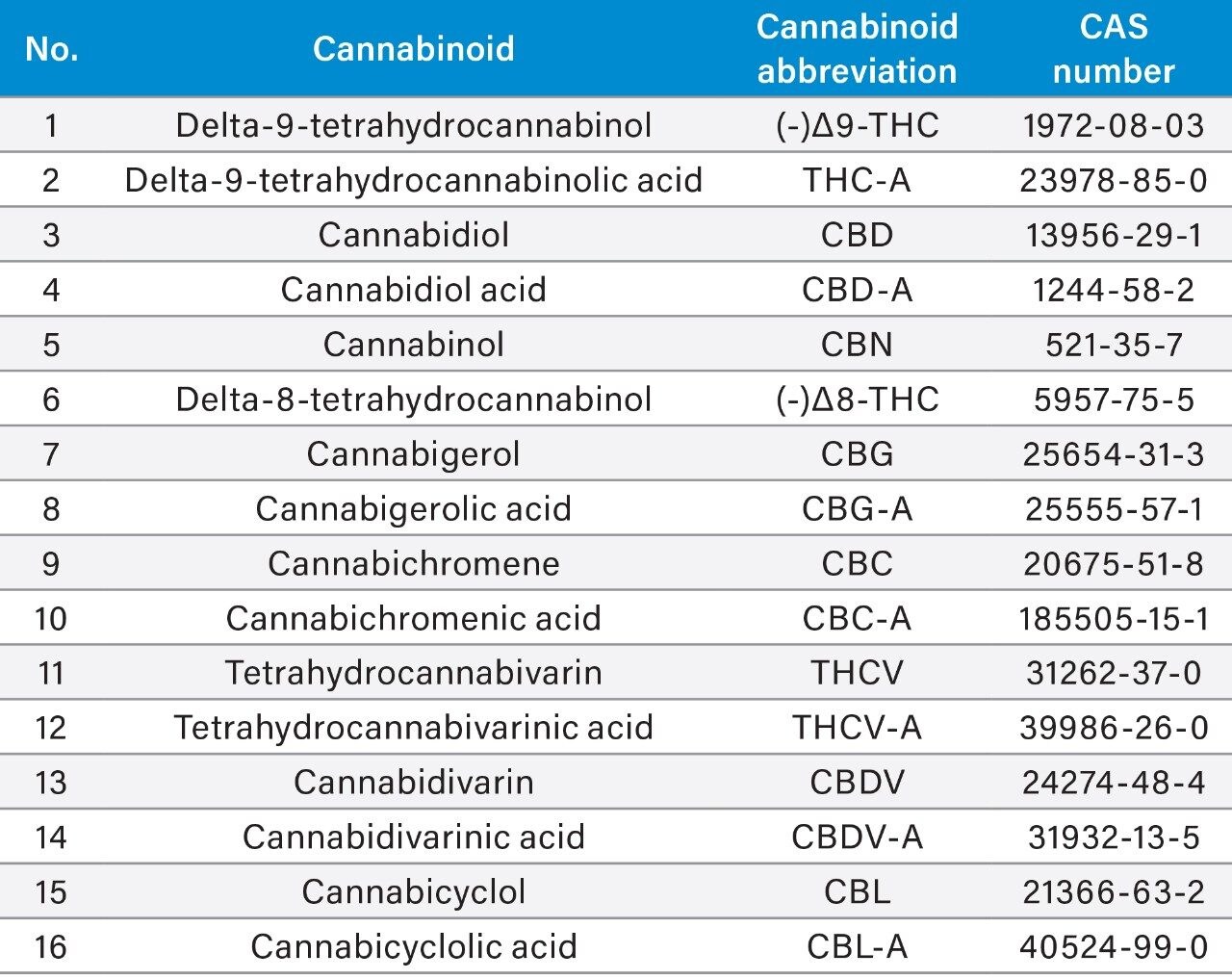
US DEA exempt reference standard solutions were obtained from Cerilliant Corporation (Round Rock, TX). These pre-dissolved solutions have been previously shown to be suitable for the generation of calibration curves when handled in an appropriate manner.2,3,4 Table 1 lists the cannabinoids used in this application note.
The HPLC column, flow rate, and injection volume described in the application note, “Separation of 16 Cannabinoids in Cannabis Flower and Extracts Using a Reversed Phase Isocratic HPLC Method”1 (720006426en) was scaled to UPLC method parameters as described in USP General Chapter <621> titled “System Suitability” for isocratic chromatographic separations.5 The HPLC method L/dp ratio (where L is column length and dp is particle diameter) was retained as much as possible, by maintaining the CORTECS Shield RP18, 90 Å particle chemistry and moving from a 2.7 µm 4.6 mm × 150 mm column (p/n: 186008685) to a CORTECS UPLC 1.6 µm particle size with 2.1 mm × 100 mm column (p/n: 186008694) dimensions. The injection volume was scaled proportionally to account for column length and diameter according to the equation for geometric scale-up.6
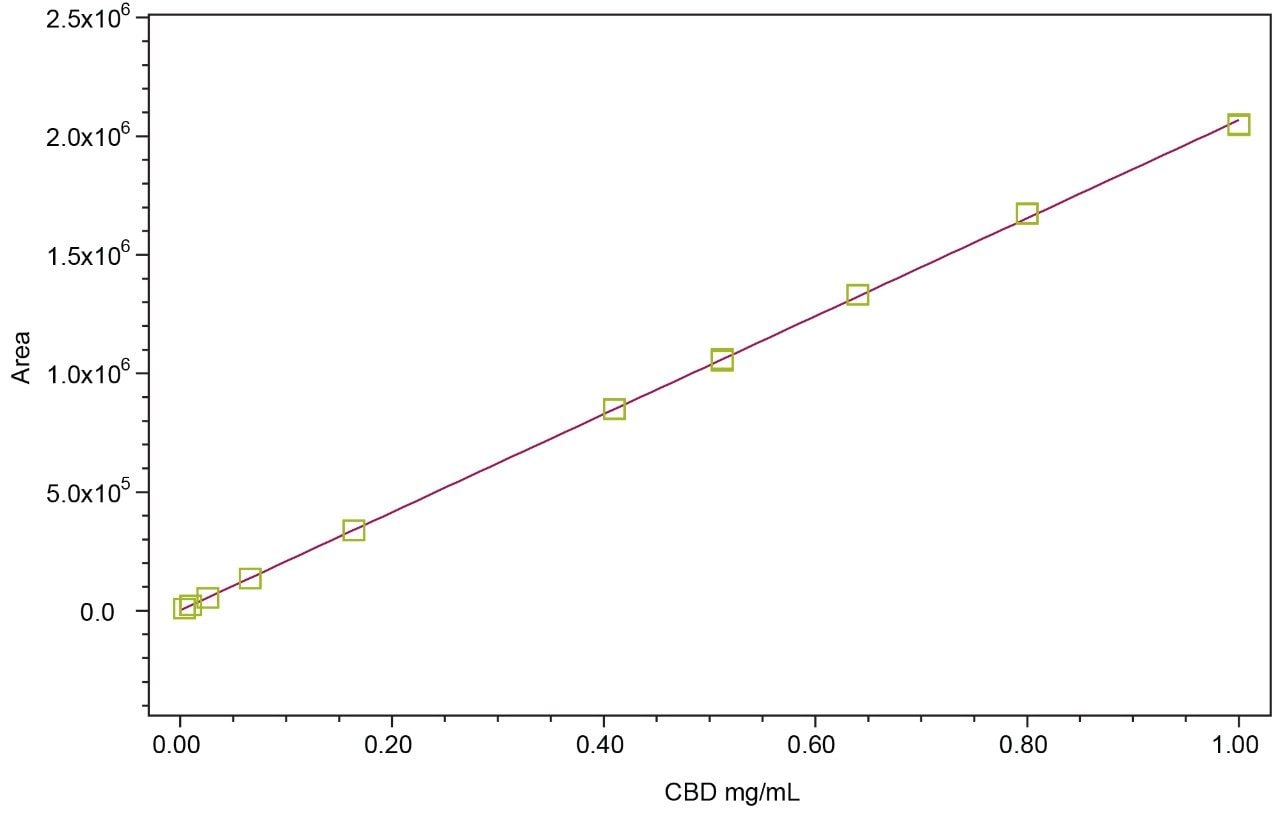
Preparation of standard curves: Linearity of primary cannabinoids (-)Δ9-THC and CBD were determined for 10 concentrations between 0.004 mg/mL and 1.000 mg/mL, prepared via serial dilution in methanol using the DEA exempt standards as a representative demonstration of method linearity.
Samples: Four representative pre-prepared cannabinoid flower and concentrate samples were obtained from a local testing laboratory in Massachusetts and one sample acquired from a hemp processing laboratory in Vermont. Samples were prepared by the manufacturers as follows: For flower, a portion of homogenized plant material was added to acetonitrile or ethanol and sonicated for 20 minutes. The subsequent extract was filtered through a 0.22 µm syringe tip filter directly into a 2 mL sample vial for analysis. Concentrates were prepared similarly with isopropanol as the extraction solvent.
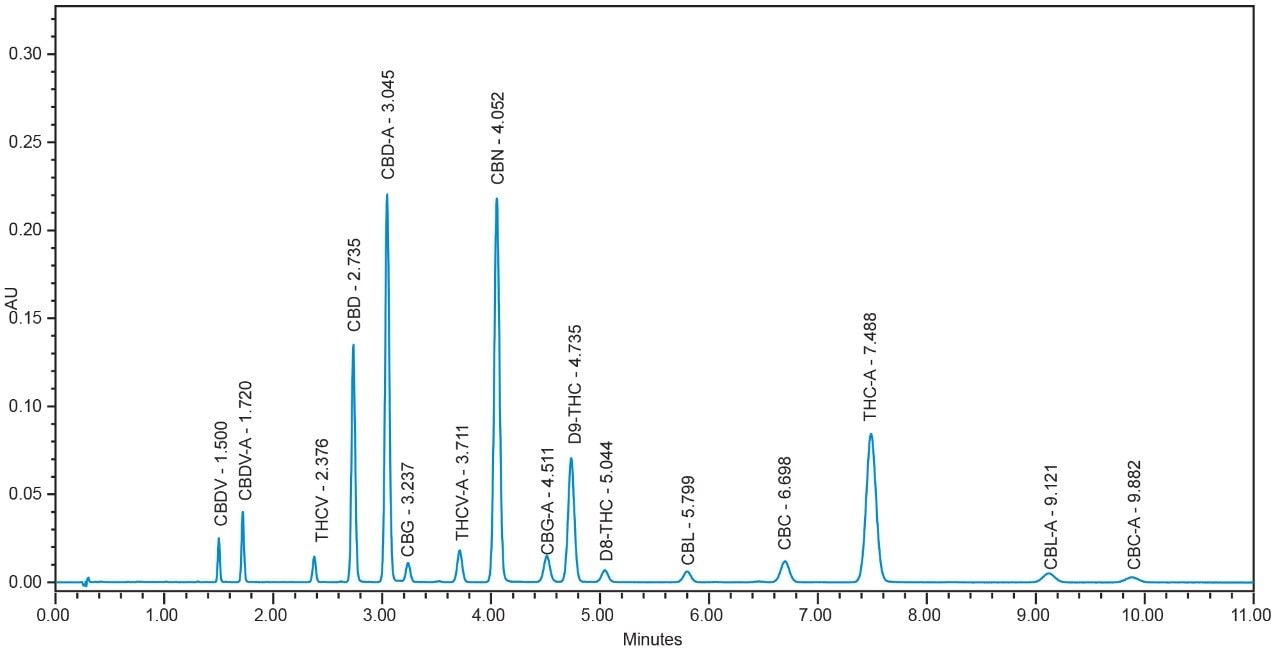
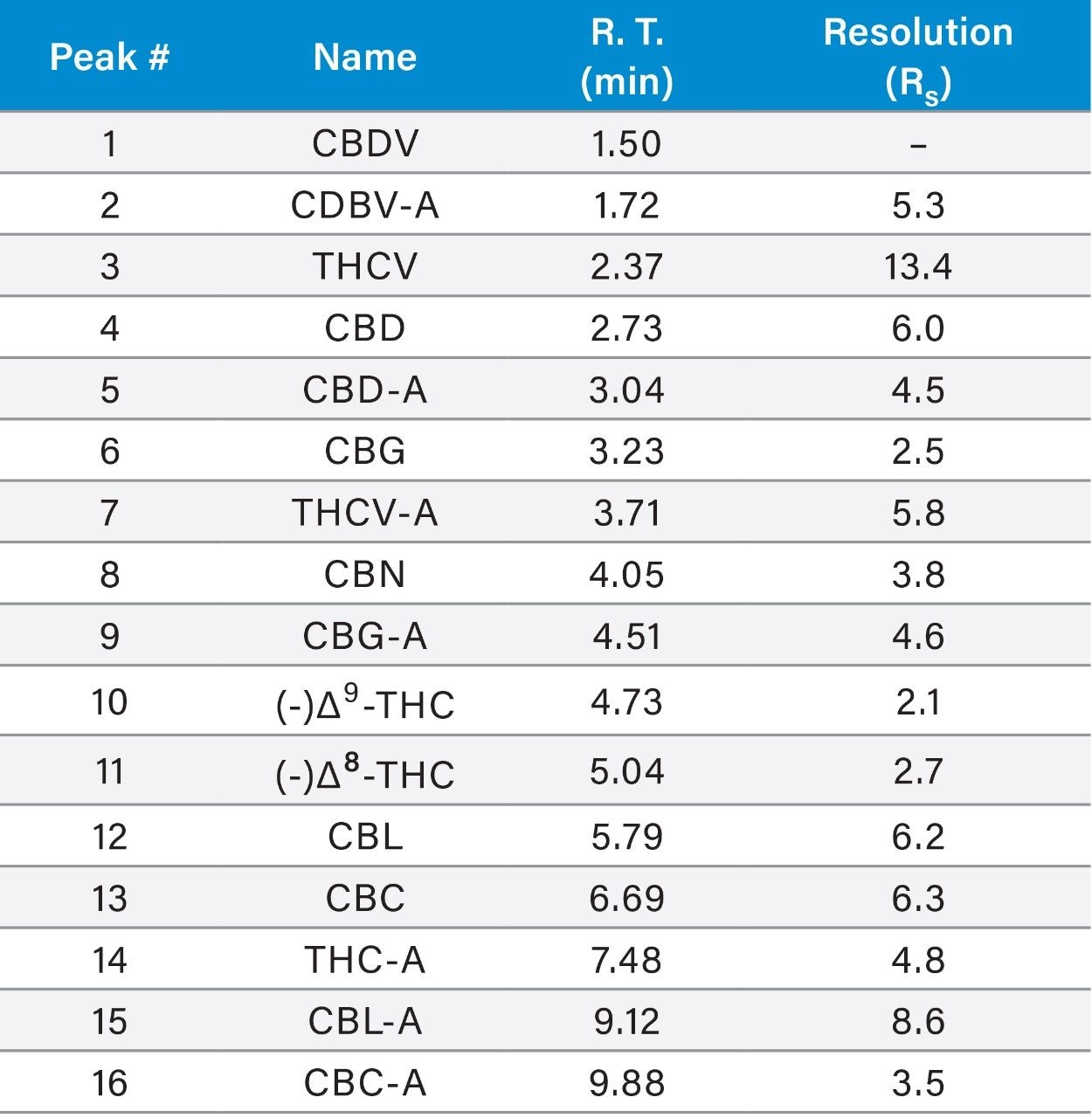
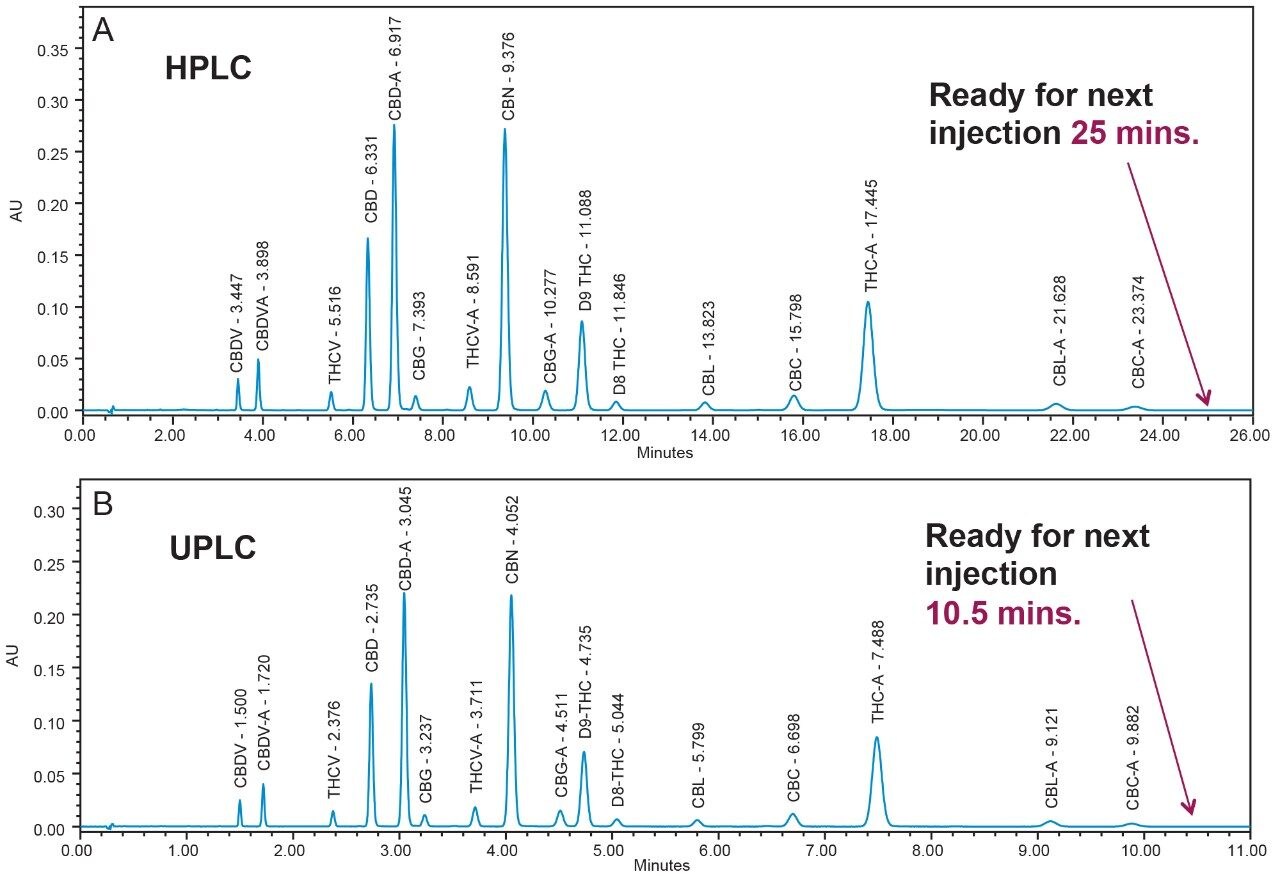
In the method presented, UPLC conditions are utilized to separate 16 cannabinoids in 10.5 minutes using 0.1% trifluoroacetic acid (TFA) in a mixture of water and acetonitrile, under isocratic conditions, combined with a CORTECS UPLC Shield RP18 Column and the ACQUITY UPLC H-Class System (Figure 1). The selectivity observed under HPLC conditions was maintained in the UPLC separation, and the resolution (Rs) of all 16 compounds was >2.0 (Table 3, Figure 2), which meets Rs recommendations for reliable quantitation.7
Multi-point calibration curves for two representative cannabinoids (CBD and (-)Δ9-THC) demonstrated good linearity at R2 >0.9998. The calibration curve for CBD is presented in Figure 3, as a representative.
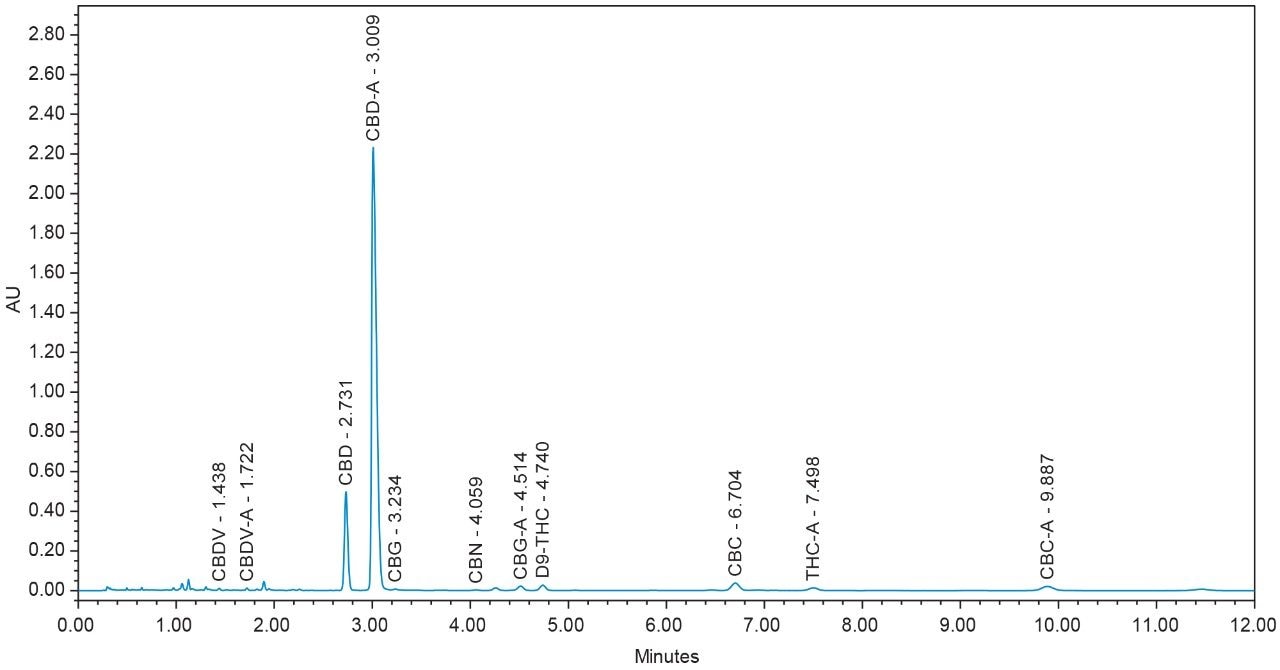
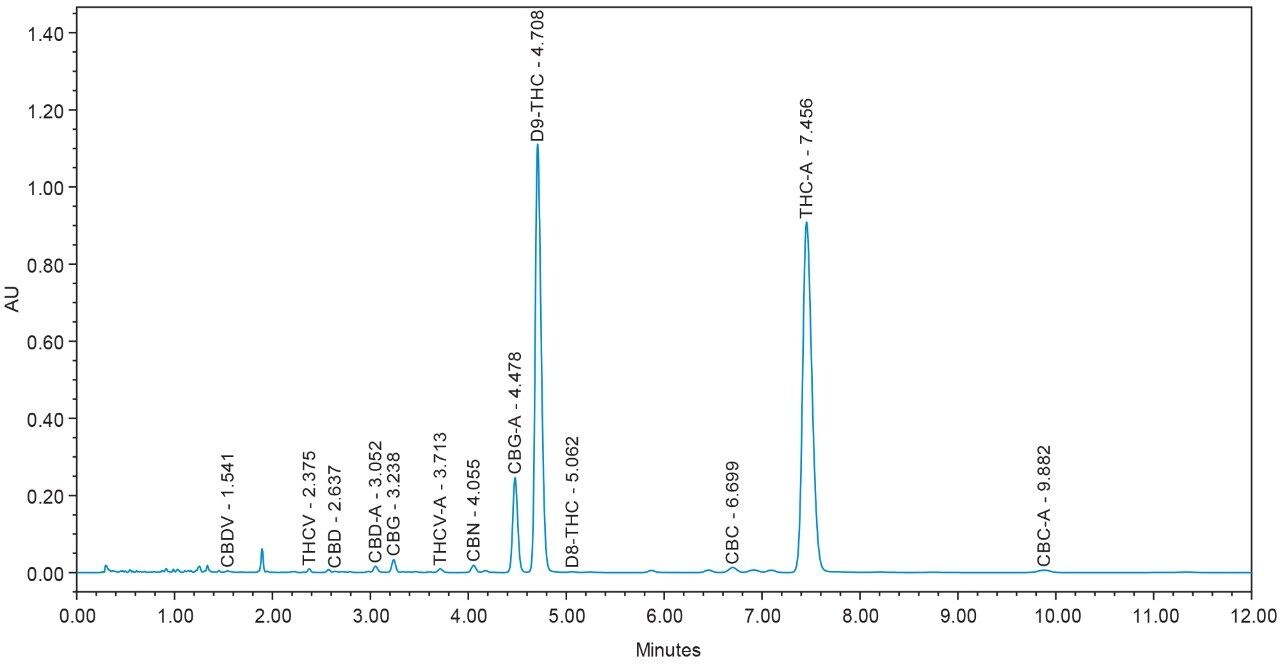
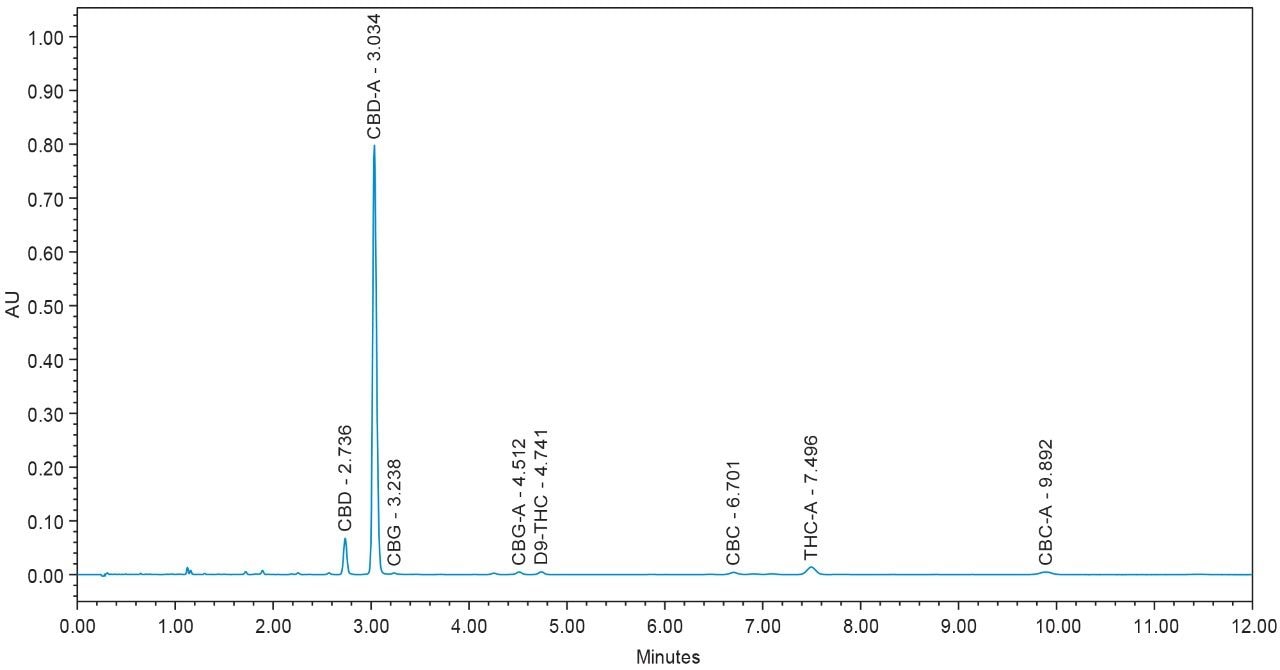
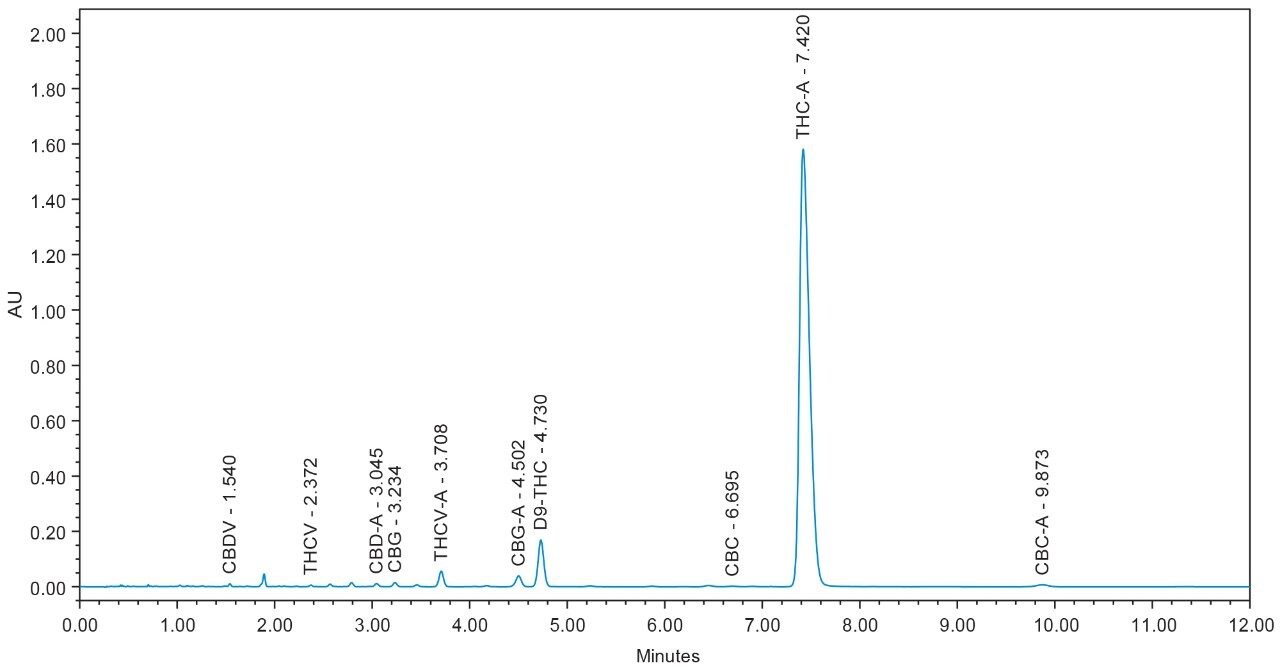
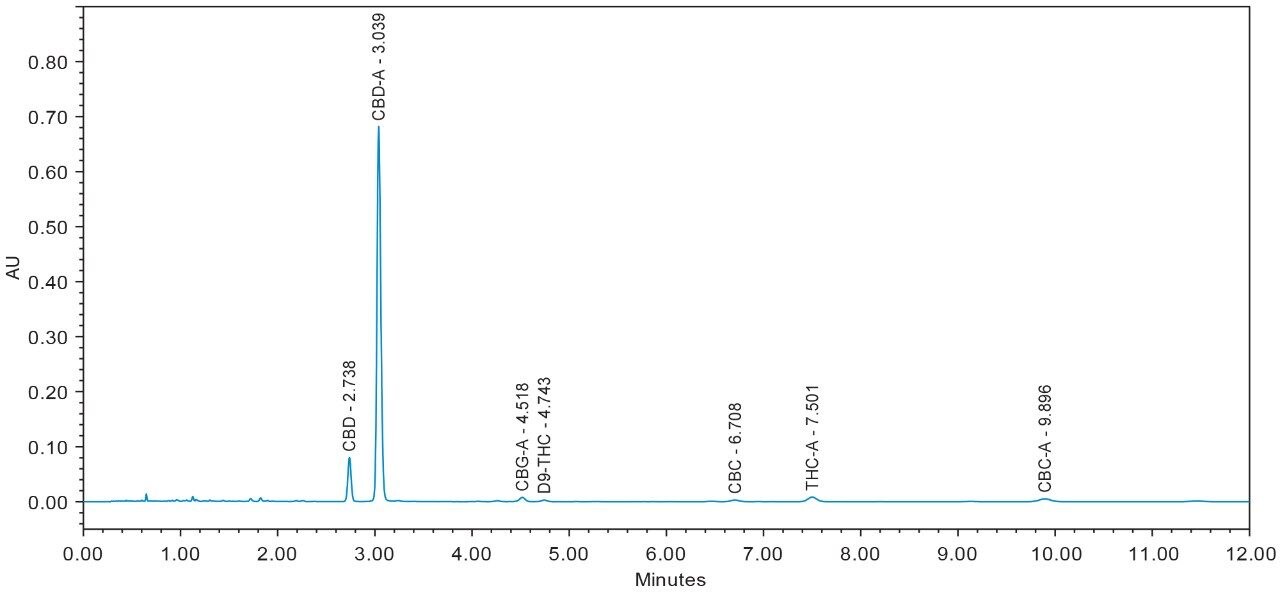
Chromatograms for flower and concentrate samples are provided in Figures 4, 5, 6, 7, and 8. The major and minor cannabinoids, as identified by injection of the cannabinoid reference standard solutions, are labeled accordingly.
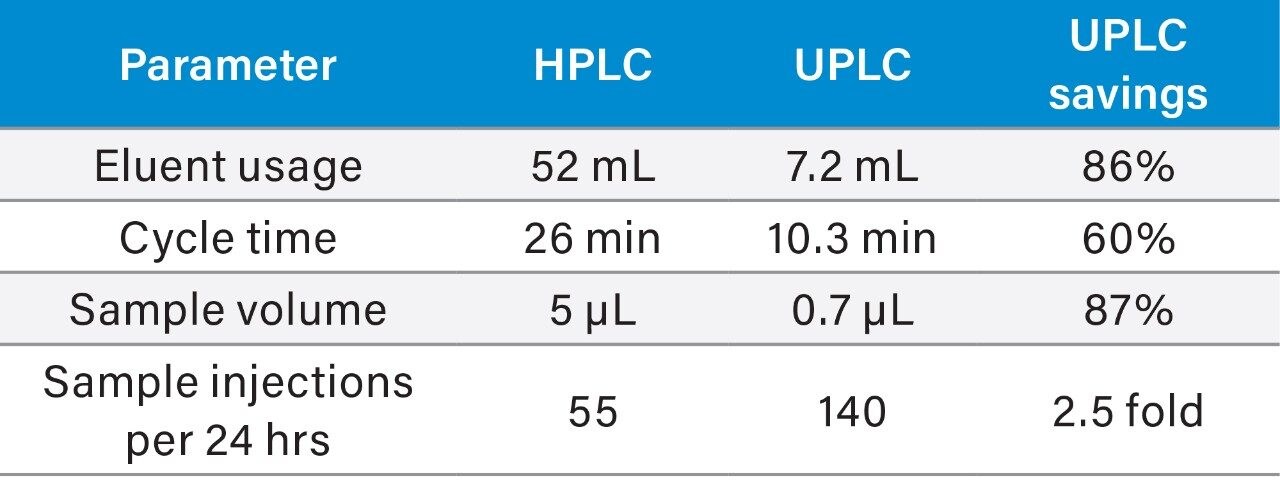
Cannabinoid separation utilizing UPLC conditions, in combination with the ACQUITY UPLC H-Class System, provide a 2.5 fold increase in the number of samples analyzed per day, plus 85% savings in eluent usage when compared to HPLC (Table 3). Analogous to the HPLC method, the separation employs the CORTECS UPLC Shield RP18 Column under isocratic conditions to further reduce injection cycle time. With the subsequent increase in productivity provided by the conversion to UPLC, the method described can be employed for separation of complex flower and concentrate samples containing major and minor cannabinoids with rapid turn-around time.
The authors thank ProVerde Labs, Milford, MA, and Cattis LLC Hardwick, VT for the donation of the prepared samples.
Waters does not support, encourage or promote the use of its products or services in connection with an illegal use, cultivation, or trade of cannabis or cannabis products. Waters products are intended to be used for cannabis related purposes only in compliance with all applicable laws in a manner that promotes public safety and/or in connection with federally approved research or state approved medical research.
720006509, February 2019