This application note demonstrates a robust and effective method for the separation, isolation, and identification of Canagliflozin and its isomeric impurities by Waters ACQUITY UPC2 SFC Investigator System, and MS detection.
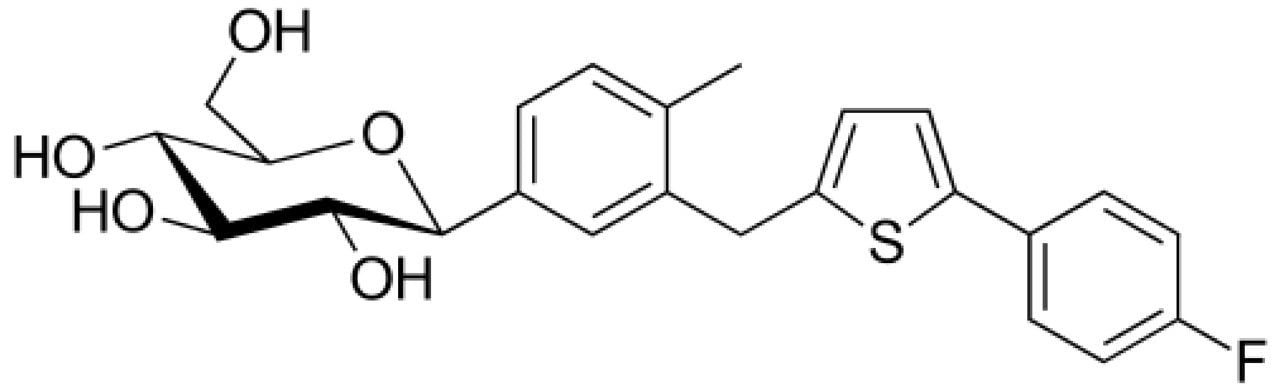
Canagliflozin is an anti-diabetic beta-isomeric drug used to improve glycemic control in people with Type 2 Diabetes. In development of generic drug for ANDA approval, synthesis of Canagliflozin often begins with materials containing sugar moieties having alpha and beta isomers in equimolar proportions. These facts serve as a potential source of undesired isomeric impurities in the final API, which must be separated and collected for their structural information. Fast turnaround time is essential for generic ANDA submissions. It is quite challenging to separate alpha and beta isomers in normal and reverse phase chromatography and hence it is difficult to purify these isomers.
In addition, SFC Investigator System is deemed by many to be a cost effective preparative/semi-preparative chromatographic technique. Due to higher diffusivity and lower viscosity of supercritical fluid, SFC Investigator System provides a three- to eight-fold faster separation than normal and reverse phase LC, resulting in a measurable increase in productivity.
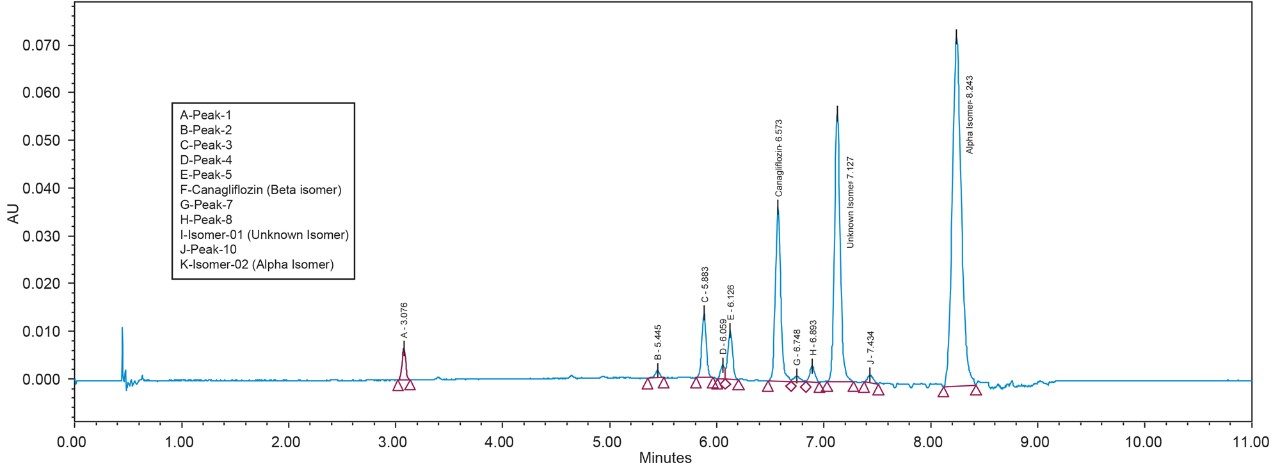
Canagliflozin and its isomeric impurities were analyzed by reverse phase (RP) chromatography. RP chromatography for such analysis takes a longer run time of 65 minutes and does not provide enough resolution for the targeted isomeric entities. With RP chromatography, it is extremely challenging to develop a method with enough separation of the conformational isomers that can be scaled up to a prep system. (Rapid) screening was performed using the ACQUITY UPC2 which yielded a method that had sufficient resolution to separate the isomers. The method was then scaled up, transferred and further optimized to SFC with similar chiral chemistry to obtain a similar separation profile. The optimized method with a non-Waters chiral column of similar chemistry was used for method transfer to SFC Investigator System, achieving better resolution of the targeted peaks of interest. The transferred method with high resolution of the isomeric peaks can be used for scale up to prep SFC systems.
The SFC Investigator System can be utilized as both an analytical and semi-preparative instrument. Compared to liquid chromatography, SFC offers unique selectivity, less organic consumption and waste removal, smaller collection volume, and faster post purification dry down time. Hence, the method screened in UPC2 was transferred to an analytical column of different chemistry of similar chiral affinity that could suit the higher flow rate of SFC Investigator System when transferred. Optimization of the method in UPC2 with an analytical column saved time and higher solvent consumption before proceeding to direct method transfer to SFC investigator System.
ACQUITY UPC2 method is further transferred to SFC Investigator and optimized with low flow rate to achieve the best possible resolution of the peaks of interests. This facilitated ease of transfer of the method to higher SFC prep instruments with higher loading.
This application note demonstrates the separation, purification, and subsequent identification of Canagliflozin and its isomeric impurities from its other impurities in a shorter runtime with improved separation.
|
System: |
ACQUITY UPC2 with ACQUITY UPC2 PDA Detector |
|
Software: |
Empower 3 |
|
Detection: |
UV at 290 nm from UV range 200–430 nm (compensation reference 330–430 nm) |
|
Column: |
ACQUITY UPC2 Trefoil AMY1, 3 x 150 mm, 2.5 μm |
|
Column temp.: |
45 °C |
|
Sample temp.: |
10 °C |
|
Sample conc.: |
250 ppm |
|
Injection volume: |
5 μL |
|
Flow rate: |
1.5 mL/min |
|
Mobile Phase A: |
Compressed CO2 |
|
Mobile Phase B: |
0.1% Trifluoro acetic acid in methanol:isopropyl alcohol (50:50) |
|
Run time: |
11 min |
|
ABPR pressure: |
2000 psi |
|
Gradient: |
5% B for 0.8 minute, ramp to 50% of B in 7 minutes, hold at 50% B for 1 minute, and return to 5% B in 0.5 minutes to equilibrate up to 11 minutes |
|
Diluent: |
Acetonitrile |
|
System: |
ACQUITY SQ Detector |
|
Polarity: |
Positive |
|
Software: |
Empower 3 |
|
Cone voltage: |
30 V |
|
Capillary voltage: |
3 KV |
|
Desolvation temp.: |
300 °C |
|
Desolvation gas: |
550 L/Hr |
|
Cone gas: |
20 L/Hr |
|
Make up solvent: |
Methanol with 0.1% acetic acid |
|
Make up solvent flow: |
0.3 mL/min |
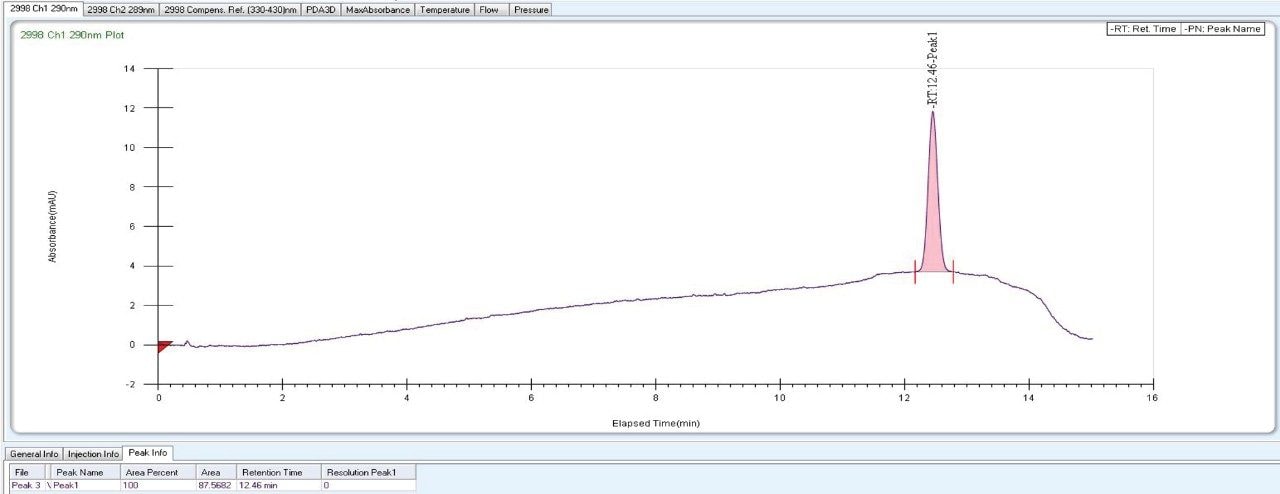
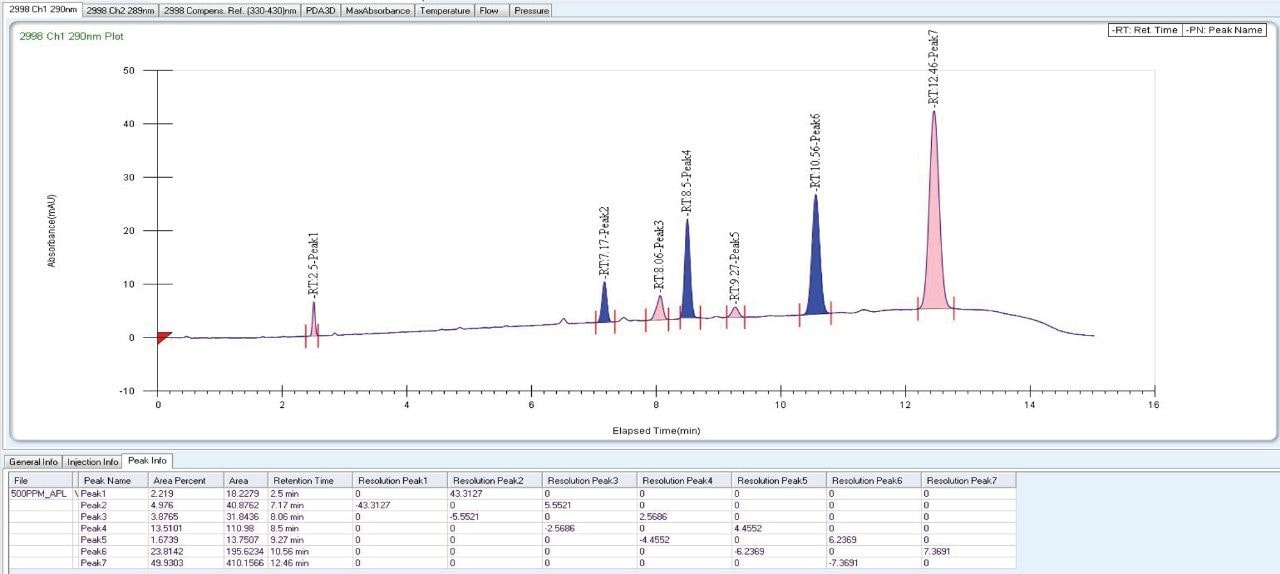
As the data (Figure 4) suggests, a quick screening of all Trefoil Column chemistries in UPC2, provided the choice for selection of the chiral chemistry for best selectivity. A quick screening with Trefoil chiral Column chemistries on UPC2 system eliminates the necessity of screening with wide range of non-Waters chiral column chemistries on the SFC Investigator System which helps save time and solvent consumption. This allowed for the simultaneous development of a robust UPC2 method that can be efficiently used for testing isomeric Canagliflozin compounds in the QC environment. The selected Trefoil chiral chemistry provided the choice of selection of Diacel chiral column chemistry for method optimization. Method optimization with Diacel chiral column in ACQUITY UPC2 further provided a suitable chromatographic condition for Diacel column with lesser solvent consumption. The method was further transferred and finalized with minimum optimization in the SFC Investigator System suitable for semi prep level collection of the peaks of interest.
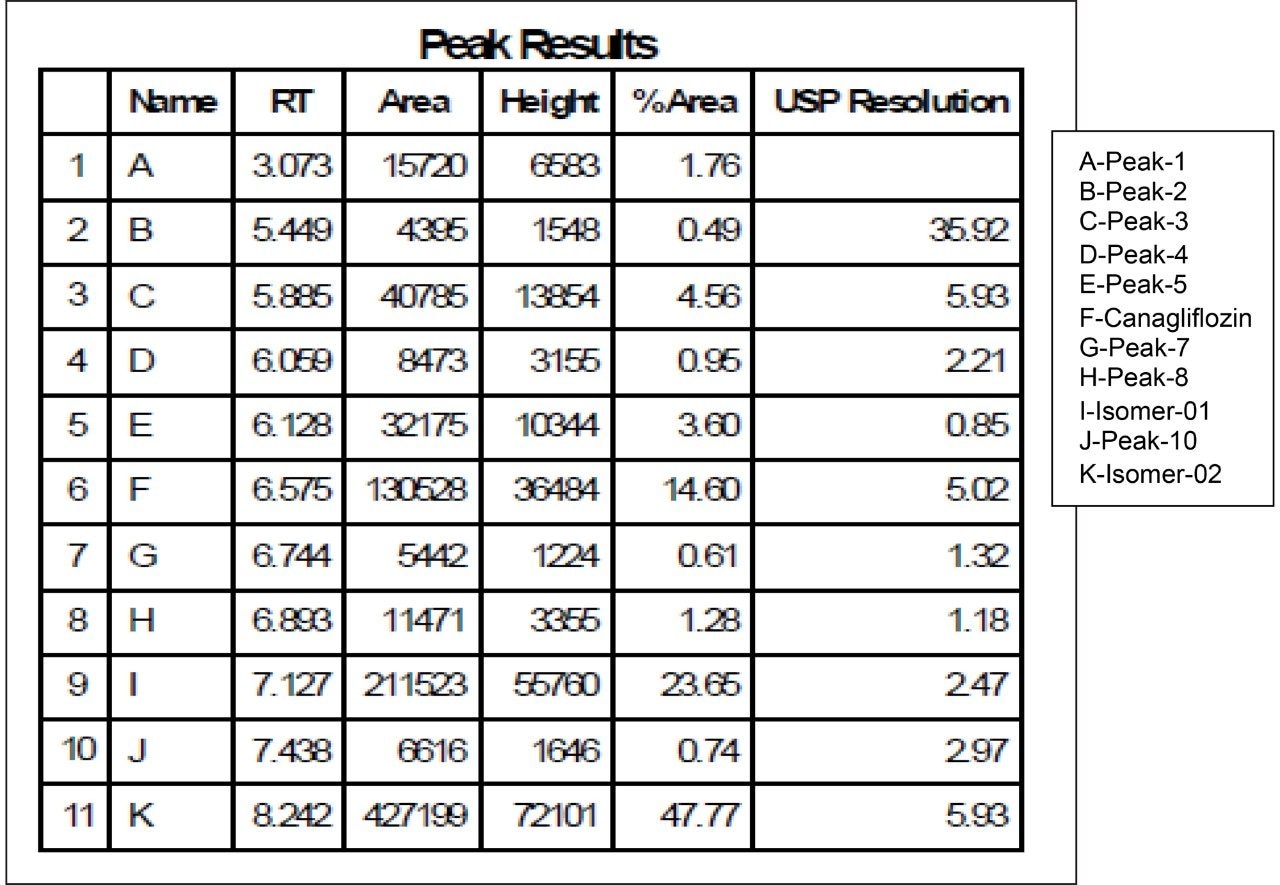
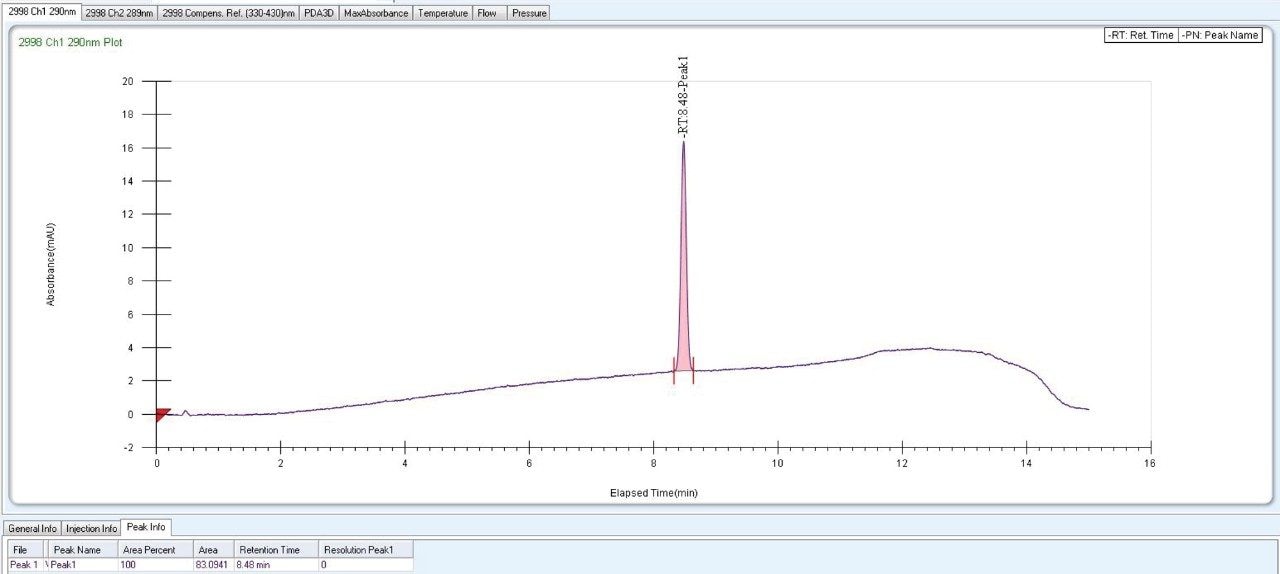
As Trefoil chiral chemistries are specific to UPC2 and analytical dimension is not yet available commercially, scale up of the method needed prior optimization with Diacel column of similar chiral chemistry that could be used for direct method transfer to the SFC Investigator. This strategy of optimizing the method with analytical columns of similar chemistry in UPC2 shortened the optimization time and solvent consumption. The method is further screened with similar column chemistry like AD-H and AD-3 analytical columns in UPC2, which will suit the higher flow rate and serve the preparative approach upon method transfer. Diacel Chiralpak AD-3 (Figure 5) was selected as the column of choice from the obtained chromatographic data.
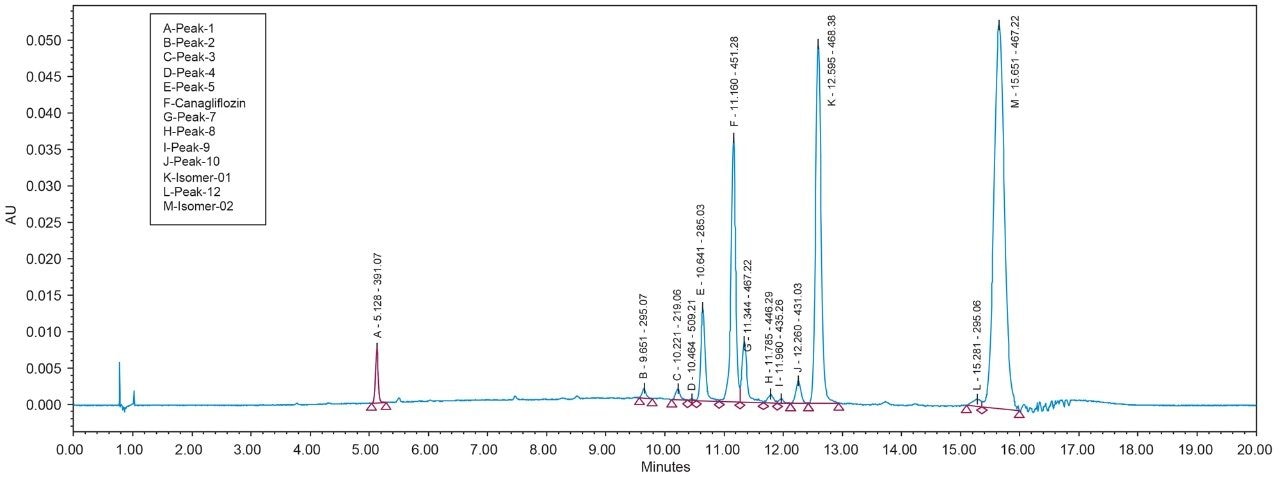
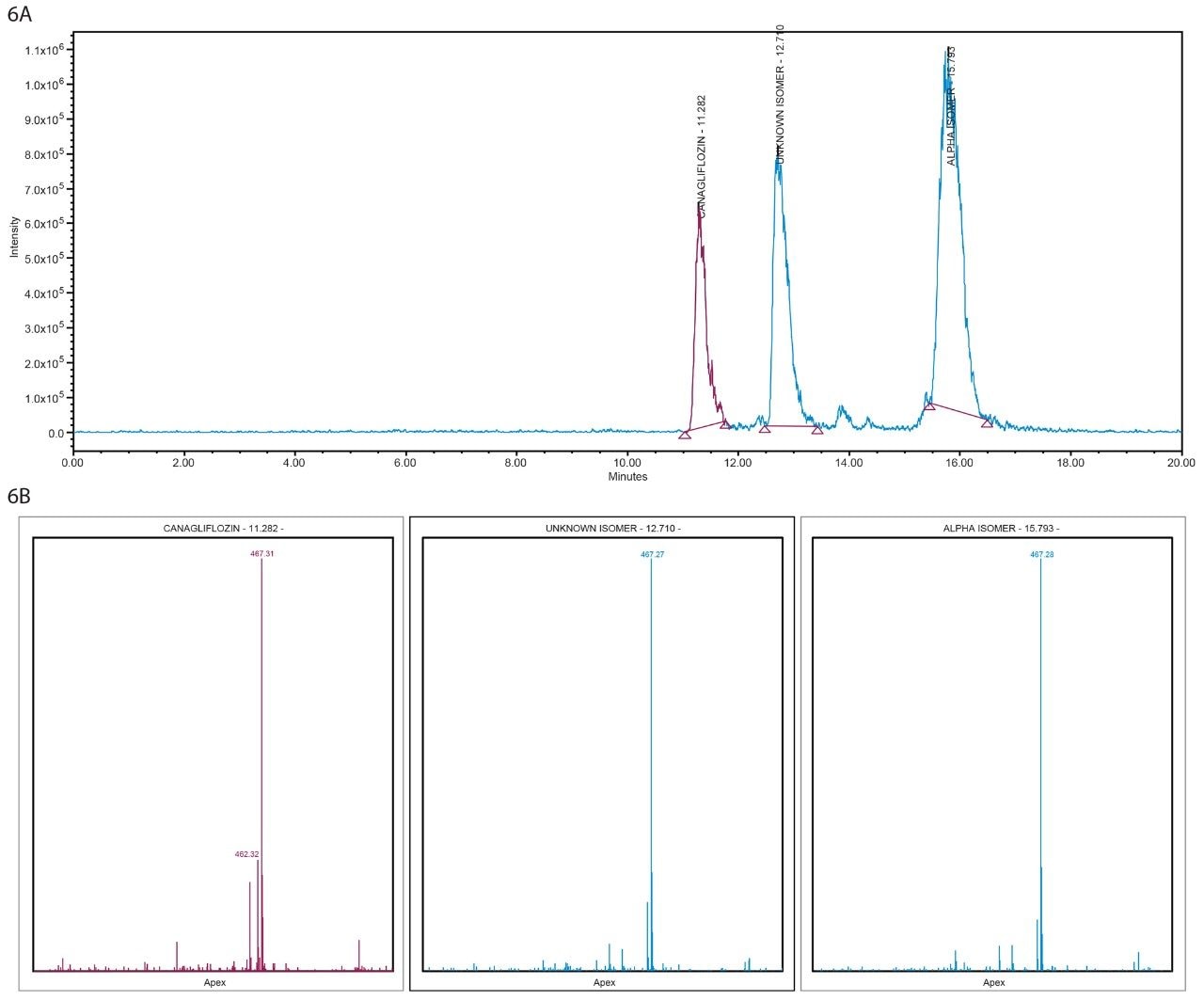
Figure 6A. MS spectra and XIC of 467 m/z in Diacel ChiralPak column from ACQUITY UPC2 interfaced with SQD.
Figure 6B. Mass Analysis plot in Diacel ChiralPak column from ACQUITY UPC2 interfaced with SQD.
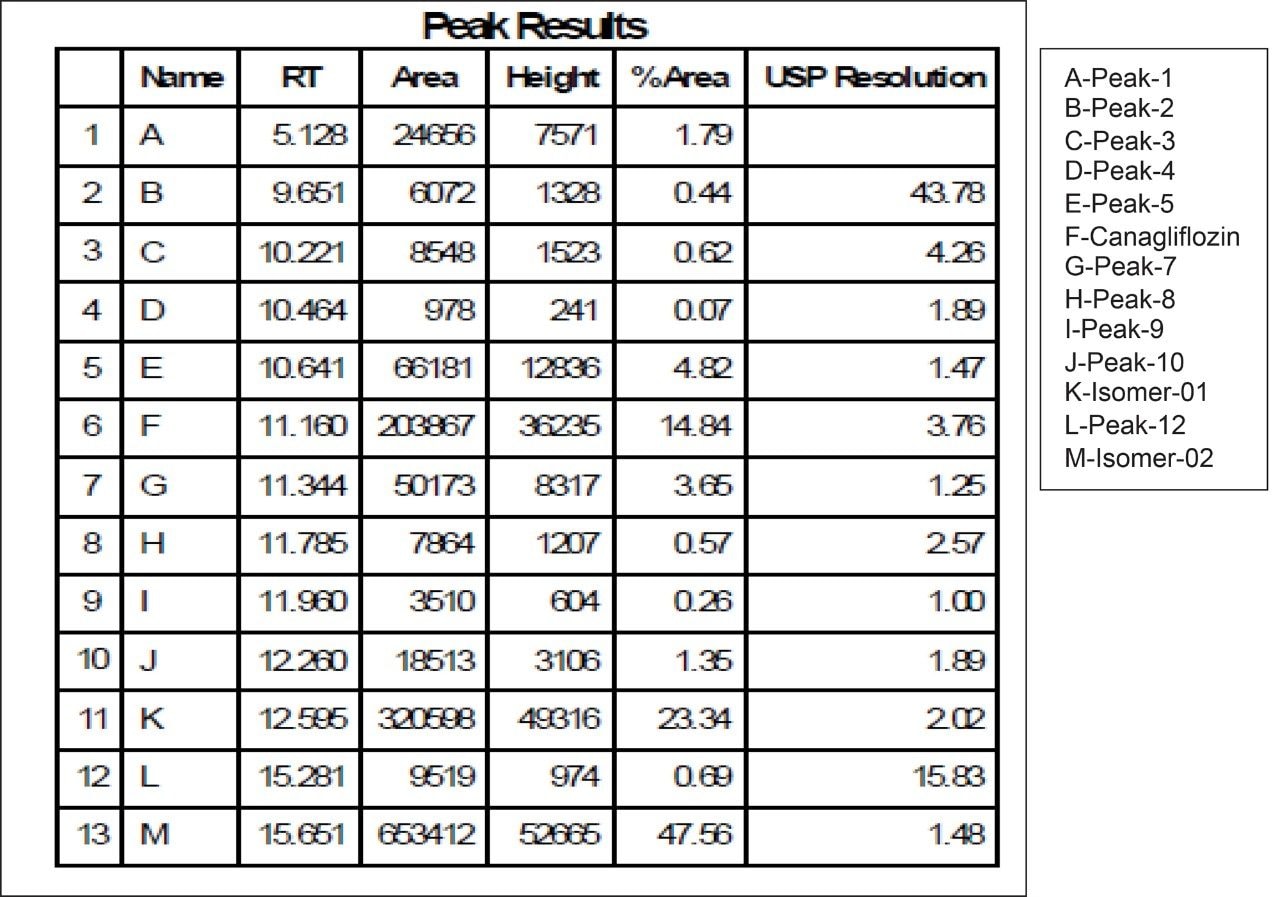
Based on the chromatographic results of UPC2 with the analytical column, the method transfer strategy is undertaken by SFC Investigator System. Keeping the chromatographic parameters the same, optimization of the method was done to achieve a lesser runtime of 15 minutes in SFC Investigator. Method transfer calculations were for the system volume difference of SFC Investigator and UPC2 System and the flowrate was optimized. Flow rate was kept as low as 5 mL/min as that would serve the purpose of easy scale up to newer SFC Prep Systems like SFC Prep 80 or SFC Prep 100 with higher loading and flow rate optimization.
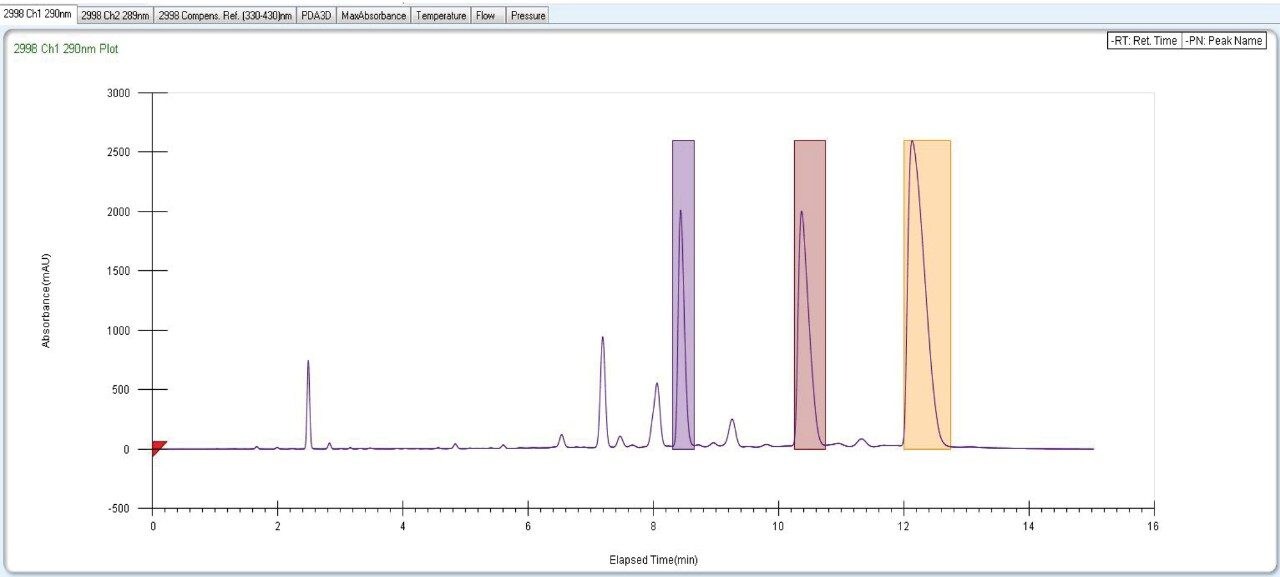
Fraction collection for peaks of interests was performed using the SFC Investigator System. The three individual collected fractions of interest were confirmed by area percent, retention time profile, and compared to UPC2 data. Recovery was found to be more than 90% when calculated based on dilution and area counts of the collected fractions (Figure 10, 11, 12).
All three peaks showed m/z 467 [M+Na] and m/z 462 [M+ NH4] (Figure 7) when analyzed by orthogonal mass detection technique in UPC2 analysis and by infusion in tandem quadrupole mass spectrometer confirming a molecular weight of 444 Da for all three isolated fractions from the SFC Investigator System.
|
System: |
ACQUITY UPC2 with ACQUITY UPC2 PDA Detector |
|
Software: |
Empower 3 |
|
Detection: |
UV at 290 nm from UV range 200–430 nm (compensation reference 330–430 nm) |
|
Column: |
Diacel ChiralPak AD-3 (4.6 x 150 mm column, 3 μm) |
|
Column temp.: |
45 °C |
|
Sample temp.: |
10 °C |
|
Sample conc.: |
500 ppm |
|
Injection volume: |
5 μL |
|
Flow rate: |
1.5 mL/min |
|
Mobile phase A: |
Compressed CO2 |
|
Mobile phase B: |
Ethanol: Isopropyl Alcohol (50:50) |
|
Run time: |
20 min |
|
ABPR pressure: |
2000 psi |
|
Gradient: |
5% B for 1.2 minute, ramp to 50% of B in 11 min, Hold at 50% B for 2 min, and return to 5% B in 2 min to equilibrate up to 20 min |
|
Diluent: |
Acetonitrile |


720006101, October 2017