
This application note demonstrates the benefits of the ACQUITY QDa Detector for method transfer and routine analysis of isoflavones in dietary supplements. The focus of this application note is on the qualitative data analysis.
LC method development is always a challenging task, especially for dietary supplements from plant sources, as the target compounds are often structurally similar to each other, and the standards are not always commercially available. The UV/Vis detection method may not provide adequate detection selectivity when needed, which makes the method development and method transfer process time consuming.
Recently, an industry standard isoflavone method1 was successfully transferred onto a Waters ACQUITY Arc UHPLC System equipped with a 2998 PDA Detector.2 The analysis time per injection was shortened from 74 minutes to 18 minutes. During the method transfer, an ACQUITY QDa Mass Detector was simultaneously used, which helped to shorten the method development and transfer time.
The ACQUITY QDa Mass Detector is a single quadruple mass spectrometer (MS) detector designed for routine analysis. It comes factory pre-optimized, so little MS expertise is required to operate it. The QDa is ready to operate minutes after pressing the power button. It fits neatly into Waters Alliance HPLC, ACQUITY Arc UHPLC, and ACQUITY UPLC stacks, and it can be controlled by either Empower CDS or MassLynx MS software.
This application note demonstrates the benefits of the ACQUITY QDa Detector for method transfer and routine analysis of isoflavones in dietary supplements. The focus of this application note is on the qualitative data analysis. The benefits for the quantitative data analysis will be evaluated in a future application note.3 The structures of isoflavones analyzed in this study are in Figure 1.
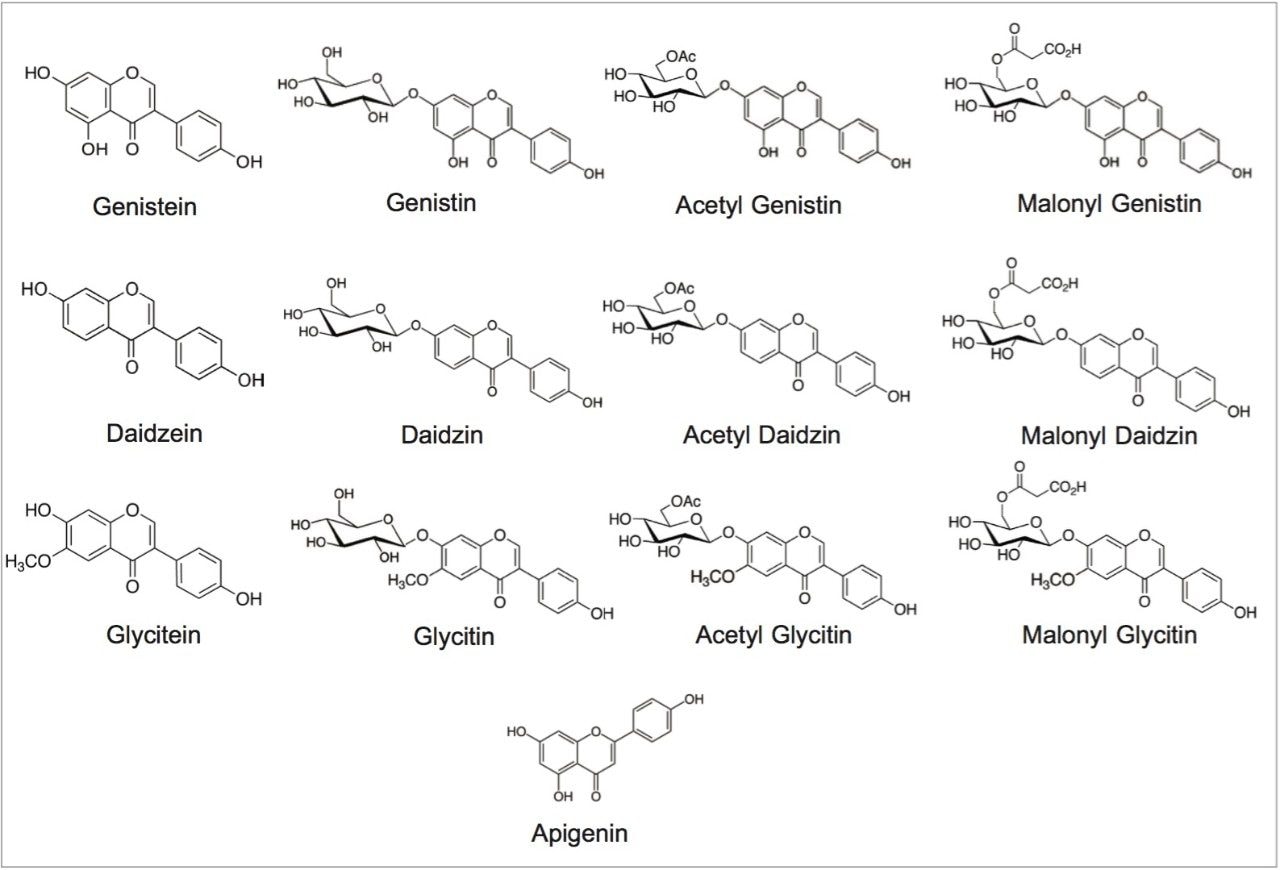
The standards: daidzin, glycitin, genistin, daidzein, glycitein, genistein, and apigenin, were purchased from ChromaDex (Irvine, CA) and INDOFINE Chemical (Hillsborough Township, NJ). Defatted powdered Soy RS was purchased from US Pharmacopeia (Rockville, MD). NIST SRM 3238 was purchased from NIST (Gaithersburg, MD). Isoflavone dietary supplement samples from major brands were purchased online.
The standard and sample solutions were prepared the same way as in the USP isoflavone method.1 Sample solutions were further diluted with acetonitrile/water mixture (2/3 by volume) to various levels to fit into the calibration range (Table 2). The concentration of the internal standard was maintained at 4 ppm.
|
UHPLC system: |
ACQUITY Arc |
|
Detector: |
2998 PDA |
|
Software: |
Empower 3 |
|
Column: |
CORTECS C18 2.7 μm, 3.0 x 100 mm |
|
Column temp.: |
30 °C |
|
Mobile phase A: |
Water with 0.1% formic acid |
|
Mobile phase B: |
Acetonitrile with 0.1% formic acid |
|
Injection volume: |
2.0 μL |
|
Flow rate: |
1.08 mL/min |
|
Run time: |
18.0 min |
|
UV detection: |
260 nm |
|
UV resolution: |
1.2 nm |
|
Time(min) |
Flow rate(ml/min) |
%A |
Curve |
|---|---|---|---|
|
Initial |
1.08 |
90 |
6 |
|
14.40 |
1.08 |
70 |
6 |
|
14.50 |
1.08 |
10 |
6 |
|
15.20 |
1.08 |
10 |
6 |
|
15.40 |
1.08 |
90 |
6 |
|
18.00 |
1.08 |
90 |
6 |
|
MS system: |
ACQUITY QDa (Performance) |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
0.8 kV |
|
Cone voltage: |
15 V |
|
Probe temp.: |
600 °C |
|
SIR masses: |
(Table 1) |
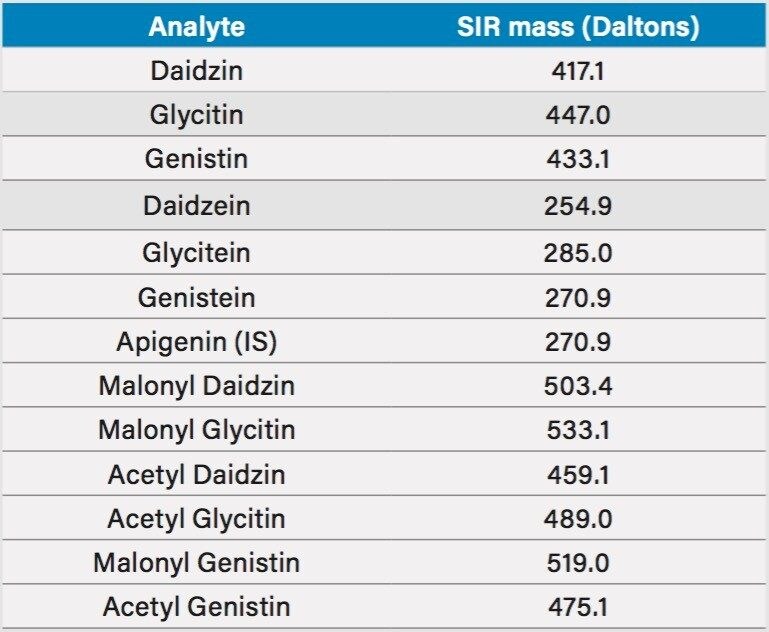
Little MS expertise is required to operate the ACQUITY QDa Detector. In this study, the factory default instrument parameters were used without any modification. The only change that was made to facilitate the MS detection was that the mobile phase additive was changed from phosphoric acid to formic acid. The mass spectra of the seven isoflavone standards (daidzin, glycitin, genistin, daidzein, glycitein, genistein, and apigenin) were obtained under the UHPLC conditions (see Experimental section), and it was confirmed that their molecular ions were the most intense ions. Individual standards of the acetyl and the malonyl isoflavones, acetyl daidzin, acetyl glycitin, acetyl genistin, malonyl daidzin, malonyl glycitin, and malonyl genistin, were not commercially available. Their mass spectra were obtained from the USP reference materials (USP defatted powdered soy RS), which contained all the acetyl and the malonyl isoflavones. The molecular ions of these compounds are listed in Table 1 and were used in the MS SIR setup.
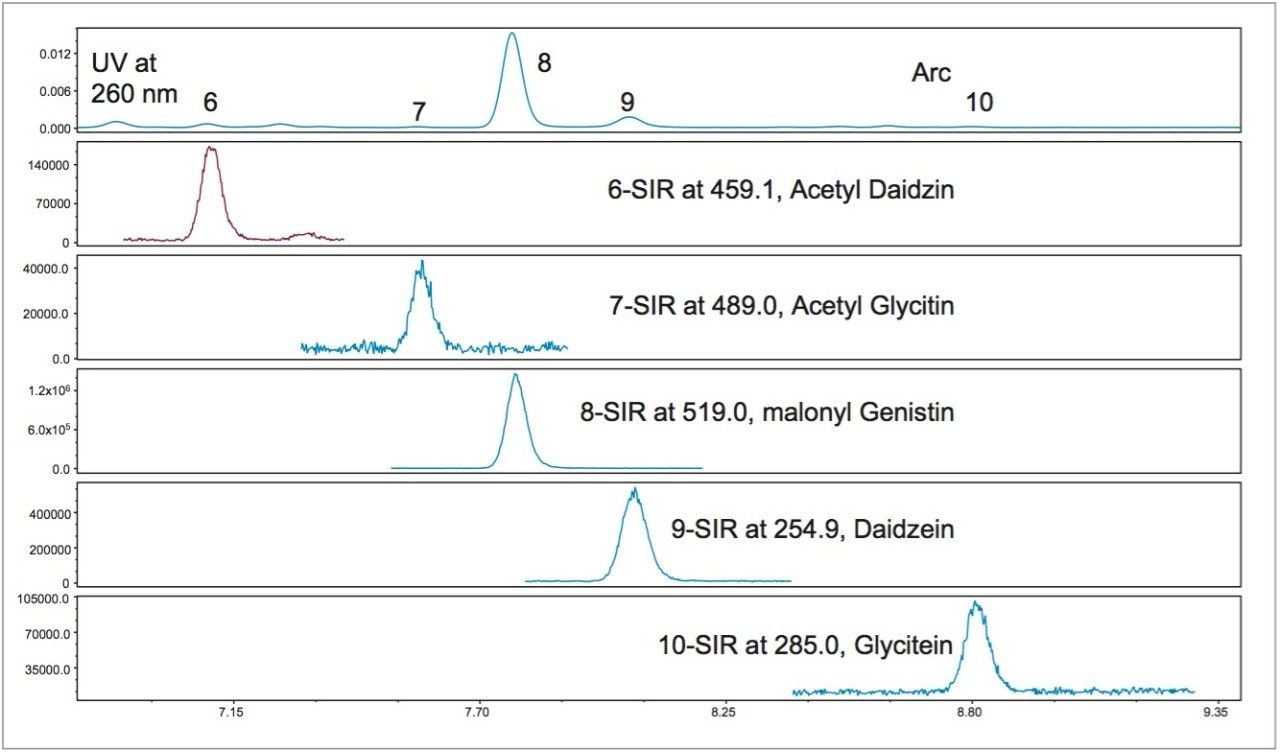
MS greatly simplifies the peak identification. The original USP method uses a pattern matching method and a reference material (USP defatted powdered soy RS) for the acetyl and the malonyl isoflavones peak identification in UV chromatograms, since these standards are not commercially available. Figure 2 shows the UV and the SIR chromatograms of defatted soy under the final optimized LC conditions. The SIR chromatograms of each compound are free of interference, which makes peak identification easier.
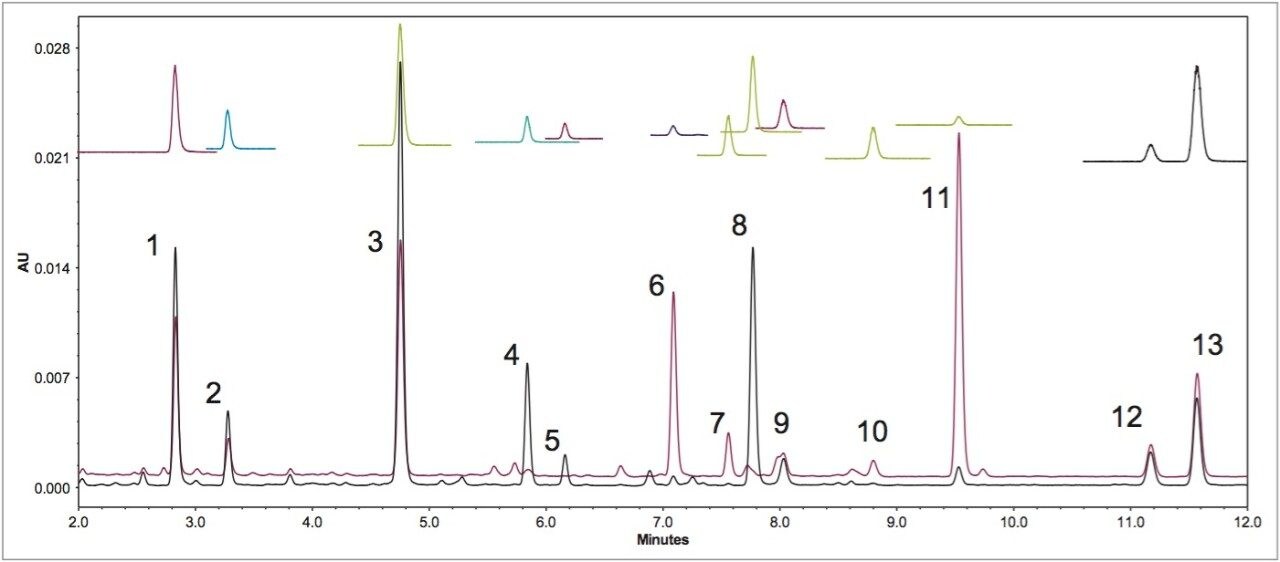
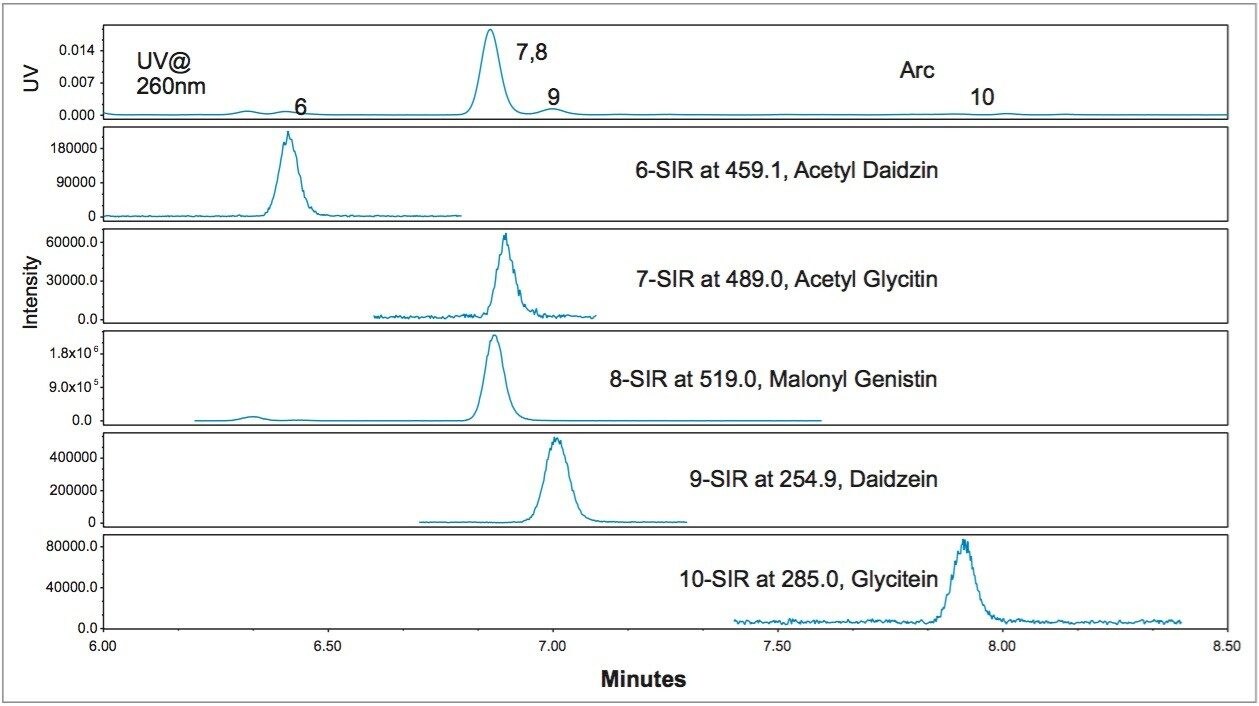
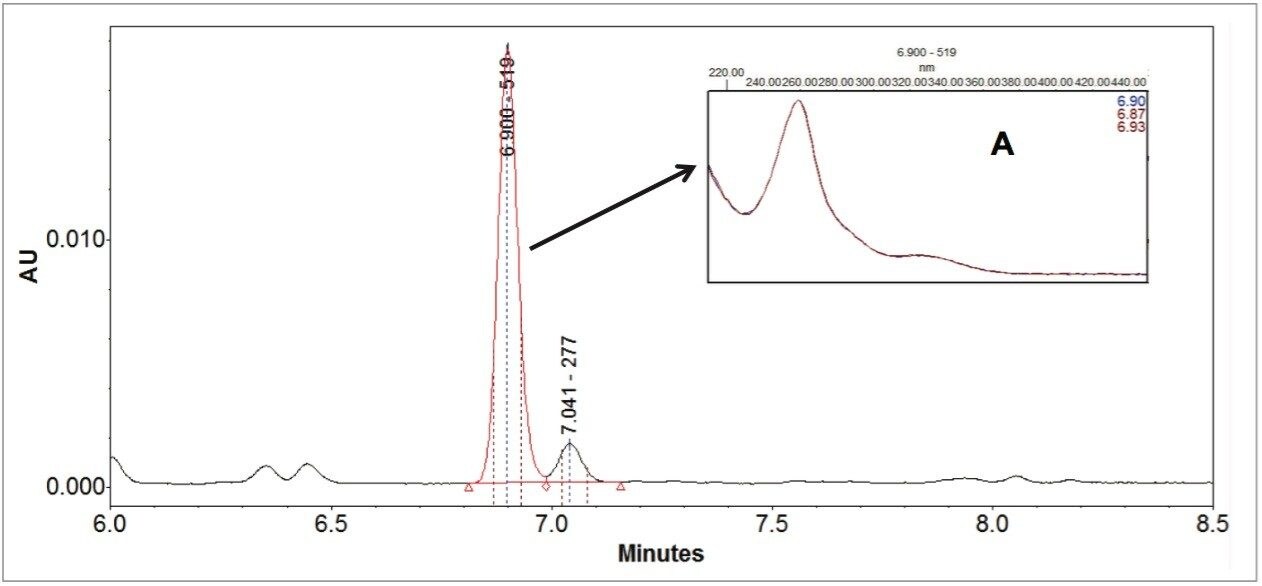
Figure 3 demonstrates the major advantage of MS data over UV/Vis spectroscopy data in confirming peak identifications. Figure 3 was obtained on a CORTECS C18 Column (2.7 μm, 3 x 100 mm), using an initial 18 minutes gradient elution program, which was converted from the USP method directly prior to further optimization.2 The SIR chromatograms of the acetyl glycitin and the malonyl genistin clearly show a co-elution. It was difficult to discern this co-elution issue from the UV (260 nm) chromatogram. Even with the PDA peak purity test (Figure 4), it was difficult since the UV spectra across the peak were identical to the peak apex UV spectrum. The ACQUITY QDa data made the co-elution issue apparent during the early stages of the method transfer, which prevented future issues or an inappropriately transferred method.
The effects of various LC conditions (column temperature and flow rate) on the isoflavones separation were evaluated to address the co-elution observed in Figure 3. Injection of one USP defatted powdered soy RS was enough to generate SIR chromatograms of all compounds for one particular LC condition, while injections of multiple standards and the reference material would be necessary for one particular LC condition if only UV (or PDA) were available. The use of the ACQUITY QDa Mass Detector reduced the number of injections that were needed for the investigation of the effects of each particular LC condition on the separation of critical compounds, and shortened the overall investigation time. Figure 5 shows the separation of the critical compounds under the final optimized conditions.
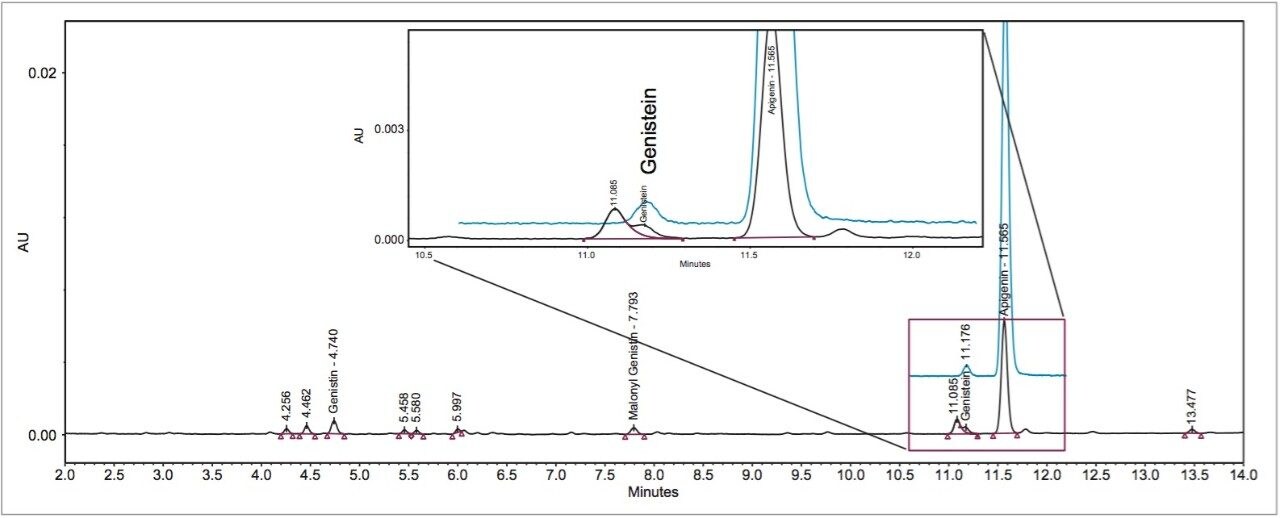
The ACQUITY QDa Mass Detector can also play an important role in routine sample analysis. Dietary supplements from plant sources often contain complex chemical matrices. The unknown matrix could interfere with the targeted compounds, and could cause error in quantitation, or even false peak assignment. Figure 6 shows the UV chromatogram of two partially co-eluting peaks for a dietary supplement sample. One of these peaks should be genistein, but it was hard to assign a peak ID because their RTs were both very close to the reference RT of genistein. The PDA data was not helpful in determining which one was genistein peak as their UV/Vis spectra were the same. Peak assignment was facile using data from the ACQUITY QDa Detector. The SIR channel of genistein (270.9 Dalton) clearly showed that the later eluting peak was genistein. This peak assignment and its purity were further confirmed and checked by inspecting the mass spectra extracted from the MS scan data at the front, the apex, and the back of the peak. Thus, the chance of false peak assignment or possible co-elution was greatly reduced.
Furthermore, Figure 6 shows how easy and simple it is to use the SIR chromatograms for the quantitation of isoflavone contents. In the UV chromatogram, one has to use a deconvolution technique to minimize the error from the interfering compound. By contrast, when using SIR chromatograms of isoflavones, there was much less chance of interference. The benefits of using MS in quantitation for isoflavone analysis will be further discussed in a separate application note.3
The MS data, simultaneously generated during the isoflavone analysis brought ease and high confidence in peak identification, and facilitated fast method transfer of the USP isoflavones method onto a Waters ACQUITY Arc UHPLC System with a 2998 PDA Detector and ACQUITY QDa Mass Detector. In comparison to PDA detection, the addition of mass detection provided an increased capability for discerning co elution issues and confirming peak identifications.
720005814, December 2016