
The USP compendial method for amoxicillin oral suspension was successfully transferred from HPLC to UPLC using the Waters Column Selectivity Chart and ACQUITY UPLC Columns Calculator. The UPLC method is approximately 70% faster than the HPLC method and affords a 92% savings in sample amount injected and mobile-phase solvent consumption. Routine column use using a phosphate buffered mobile phase at pH 5 and formulated oral suspension sample was evaluated on the ACQUITY UPLC BEH Shield RP18 column. A column wash alleviated an increase in pressure during the study and incorporating a routine wash is advocated to extend the lifetime of columns in general. After 2400 injections, the ACQUITY UPLC BEH Shield RP18 column still passed all USP suitability specifications for amoxicillin oral suspension, demonstrating that UPLC can be used to perform routine QC analysis of amoxicillin oral suspension samples without compromising column stability or separation performance.
USP compendial methods are often used as a basis for routine analysis of generically manufactured drugs. Often these methods do not take advantage of modern techniques such as sub-2 μm particle columns and UPLC. Many USP methods were also developed on older column technology, limiting efficiency of the analysis. Updating these methods to run on more current systems allows for more efficient batch analysis by maximizing sample throughput while retaining resolution.
Amoxicillin is a commonly used antibiotic that is produced generically throughout the world. Formulations vary and in this example, an oral suspension formulation is analyzed according to its USP monograph.1 The method transfer from the USP compendial method to a more modern stationary phase and subsequent analysis by UPLC is demonstrated. The robustness of the updated method is evaluated by performing a routine use evaluation study, assessing the assay suitability criteria described in the USP method to ensure long-term column stability.
|
Diluent: |
50 mM potassium phosphate, monobasic in water - pH 5.0 with potassium hydroxide |
|
Mobile Phase: |
98:2 diluent:acetonitrile |
|
Separation Mode: |
Isocratic |
|
Detection: |
UV at 230 nm |
|
USP Column: |
XBridge Shield RP18, 4.6 x 250 mm, 5 μm (USP designation L1), part number 186003010 |
|
Needle Wash: |
90:10 water:acetonitrile |
|
Sample Purge: |
90:10 water:acetonitrile |
|
Seal Wash: |
90:10 acetonitrile:water |
|
Flow Rate: |
1.5 mL/min |
|
Injection Volume: |
10 μL |
|
Diluent: |
50 mM potassium phosphate, monobasic in water - pH 5.0 with potassium hydroxide |
|
Mobile Phase: |
98:2 diluent:acetonitrile |
|
Separation Mode: |
Isocratic |
|
Detection: |
UV at 230 nm |
|
Column: |
ACQUITY UPLC BEH Shield RP18, 2.1 x 100 mm, 1.7 μm (USP designation L1), part number 186002854 |
|
Needle Wash: |
90:10 water:acetonitrile |
|
Sample Purge: |
90:10 water:acetonitrile |
|
Seal Wash: |
90:10 acetonitrile:water |
|
Flow Rate: |
0.4 mL/min |
|
Injection Volume: |
0.8 μL |
Amoxicillin oral suspension powder, reconsituted in water (50 mg/mL), made up to 1 mg/mL in diluent.
Amoxicillin standard made up to 1 mg/mL in diluent.
Samples were filtered though a 0.2 μm nylon filter (part number WAT200522), prior to analysis.
Empower 2 CDS
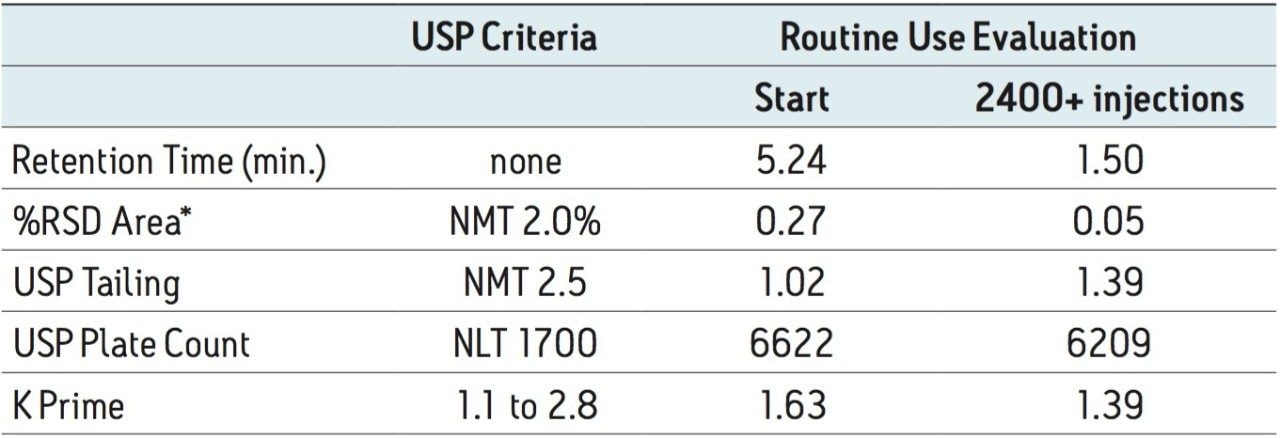
Samples were prepared according to the USP assay method guidelines for amoxicillin oral suspension. The assay preparation for amoxicillin oral suspension specifies filtering the sample through a 1 μm or finer porosity filter. Care was taken to filter samples through a 0.2 μm nylon filter to remove any fine particulates. The USP method for amoxicillin oral suspension designates the use of an L1 column and the suggested column is μBondapak C18. Using the Waters Column Selectivity Chart, a more modern L1 column, XBridge Shield RP18, was selected where direct scalability to the same UPLC column chemistry can be demonstrated. The USP compendial method was first run as described on an Alliance HPLC system using five replicate injections of both amoxicillin standard and amoxicillin oral suspension. Assay suitability criteria described in the monograph were monitored for both samples and found to be within specification (Table 1).

The USP method was then transferred from HPLC to UPLC using the ACQUITY UPLC Columns Calculator.2 Scaling was performed accounting for particle size and the column was scaled to an ACQUITY UPLC BEH Shield RP18, 1.7 μm column, maintaining the same column chemistry. Five replicate injections of both amoxicillin oral suspension and amoxicillin standard were analyzed separately. Assay suitability criteria including %RSD for peak area, k prime, USP tailing factor, and USP plate count were compared between HPLC and UPLC. A comparison of both systems is shown in Table 1, where the UPLC transferred method passes criteria in all regards. Finally, the run time of the UPLC method is 4.5 minutes compared to a 15-minute HPLC method, affording an approximate 70% savings in analysis time and 92% savings in solvent consumption and sample injected (Figure 1).
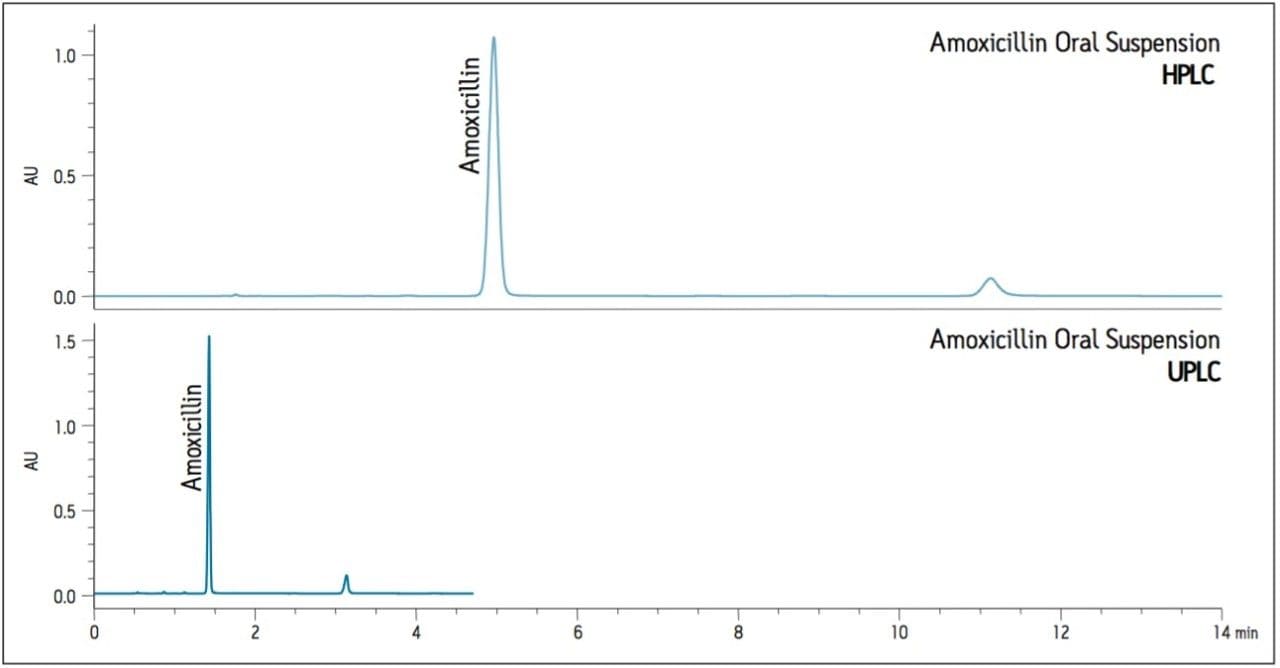
In order to evaluate the effects of using common USP mobile phases such as phosphate buffer and formulated drug samples on newer column technology, a routine use evaluation was performed on the 1.7 μm ACQUITY UPLC BEH Shield RP18 column using the amoxicillin oral suspension sample.
Amoxicillin oral suspension was analyzed using amoxicillin standard as a bracketing standard, as might be seen in a typical quality control (QC) laboratory. Five replicate injections of amoxicillin standard were followed by twenty replicate injections of amoxicillin oral suspension and this cycle of injections was repeated continuously until assay suitability criteria no longer passed. Pressure, retention time, amoxicillin peak area, k prime, USP tailing factor and USP plate count were monitored throughout the study.
Pressure remained stable at approximately 8000 psi for 500 injections, after which the pressure began to increase gradually until the maximum system pressure was reached at 1700 injections (Figure 2). The system and column were washed with 90:10 water:acetonitrile for 2 to 3 hours. The column was re-equilibrated to the method starting conditions and the pressure returned to starting levels of 8000 psi, whereby the routine use evaluation was re-started.
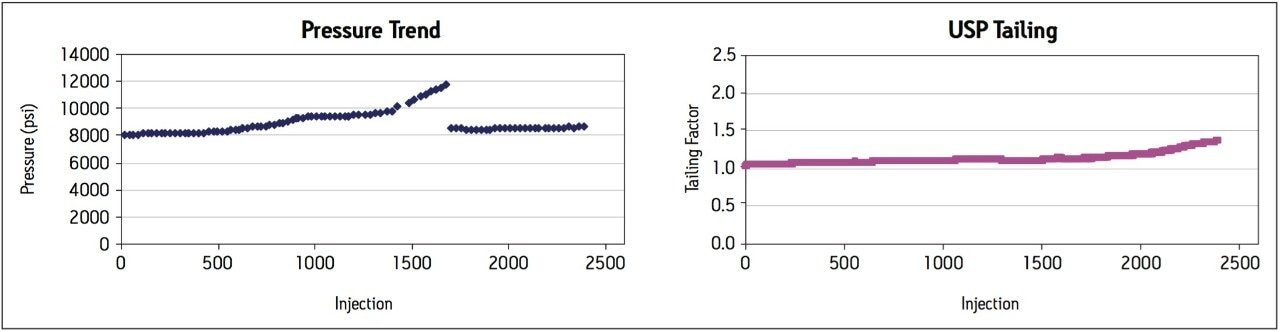
Retention factor and plate count remained within the USP assay suitability criteria throughout the study. USP tailing for the amoxicillin peak increased slightly over 2000 injections but was still well within the USP criteria of NMT 2.5. The routine use evaluation was stopped at approximately 2400 injections and system suitability results were still well within passing criteria (Table 2).
The USP compendial method for amoxicillin oral suspension was successfully transferred from HPLC to UPLC using the Waters Column Selectivity Chart and ACQUITY UPLC Columns Calculator. The UPLC method is approximately 70% faster than the HPLC method and affords a 92% savings in sample amount injected and mobile-phase solvent consumption. Routine column use using a phosphate buffered mobile phase at pH 5 and formulated oral suspension sample was evaluated on the ACQUITY UPLC BEH Shield RP18 column. A column wash alleviated an increase in pressure during the study and incorporating a routine wash is advocated to extend the lifetime of columns in general. After 2400 injections, the ACQUITY UPLC BEH Shield RP18 column still passed all USP suitability specifications for amoxicillin oral suspension, demonstrating that UPLC can be used to perform routine QC analysis of amoxicillin oral suspension samples without compromising column stability or separation performance.
720004078, April 2013