For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
This Application brief demonstrates the simultaneous and high sensitivity analysis of plasma metanephrines by LC-MS for clinical research.
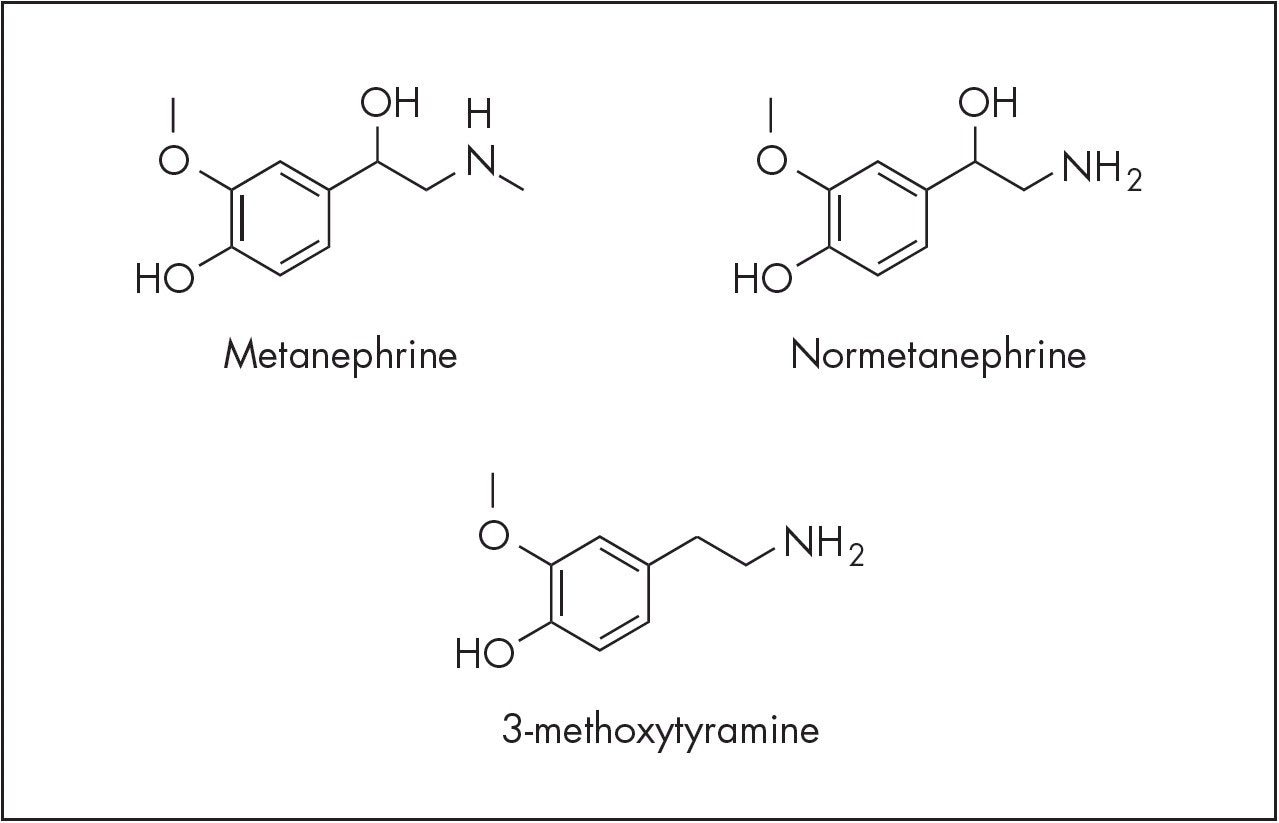
Measurement of several important plasma biogenic amines
Development of LC-MS methods for simultaneous measurement of plasma metanephrines has been challenging. Efficient sample pre-treatment strategies have yet to be defined and measurement of these molecules can be complicated by the difficult chromatographic separation of metanephrines such as 3-methoxytyramine (3-MT), from other metanephrines. Development of a good separation has proven problematic and can lead to overestimation of metanephrine levels. Many laboratories also do not measure 3-MT as the assays they use may not have the analytical sensitivity to measure this low-level analyte. To effectively study these important biogenic amines, a research method to measure metanephrine, normetanephrine and 3-MT simultaneously is needed.
|
LC System: |
ACQUITY UPLC with ACQUITY UPLC Online SPE Manager |
|
Mass Spectrometer: |
Xevo TQ-S |
|
Column: |
Atlantis HILIC, 2.1 x 50 mm, 3 μm |
|
Sample Preparation: |
ACQUITY UPLC Online SPE Manager (OSM) |
|
SPE: |
MassTrak WCX OSM Cartridge |
Plasma samples were diluted 1:1 with deuterated internal standard and then centrifuged through a 10 k MW cutoff centrifugation spin filter device to remove proteins from the sample.
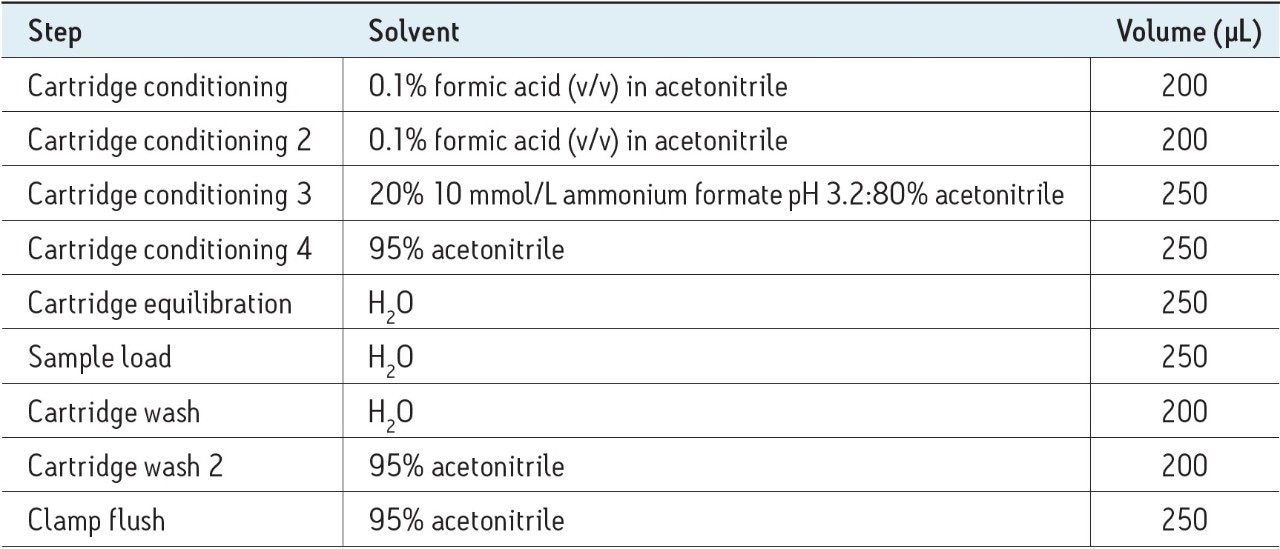
After filtration, an aliquot of sample was injected into the online SPE equipped LC-MS system. SPE was performed by the online SPE system as follows:
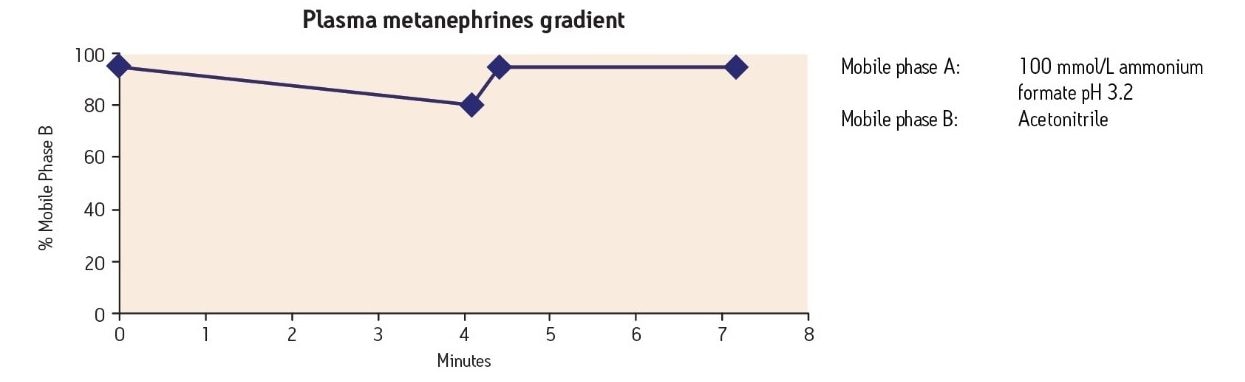
After SPE, samples were analyzed by LC-MS using the following gradient conditions:
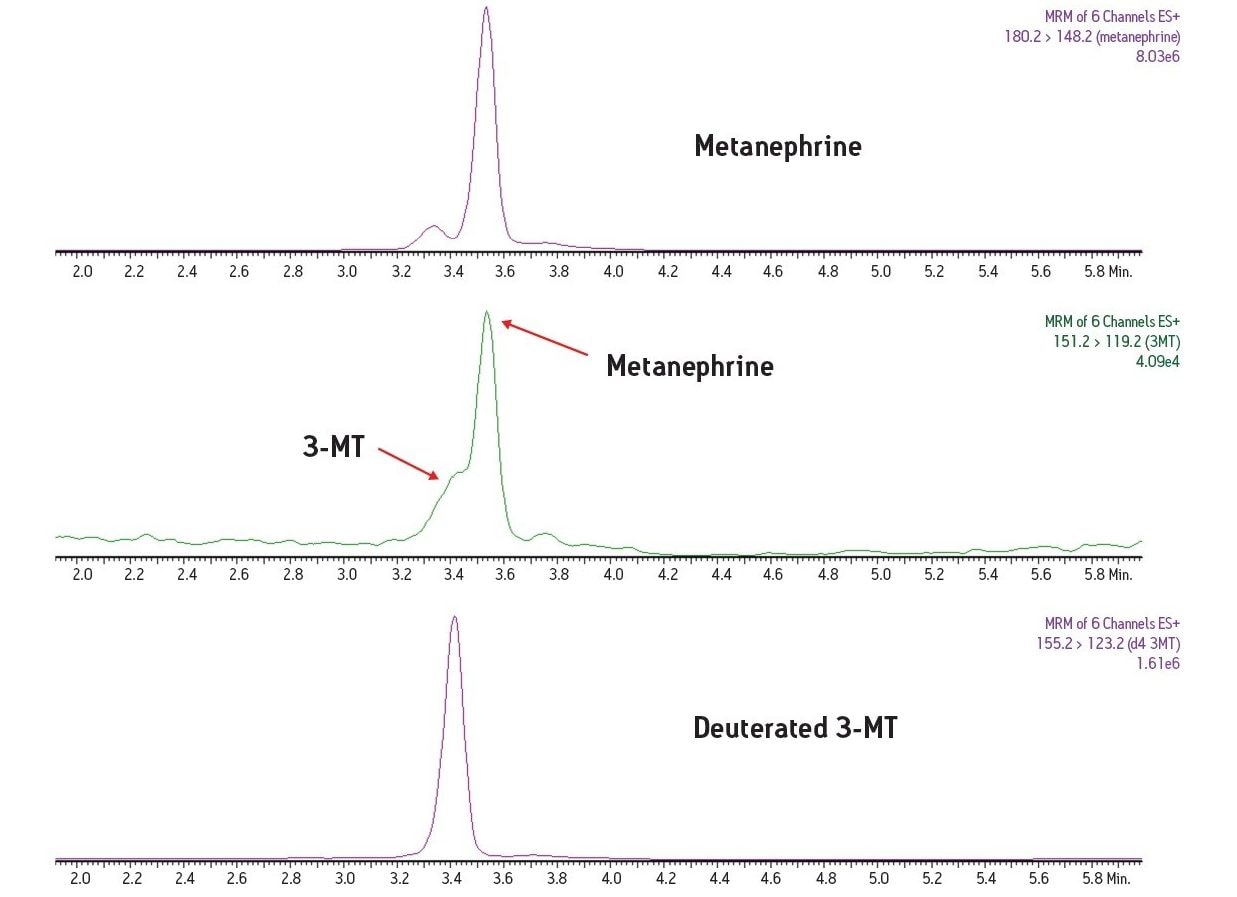
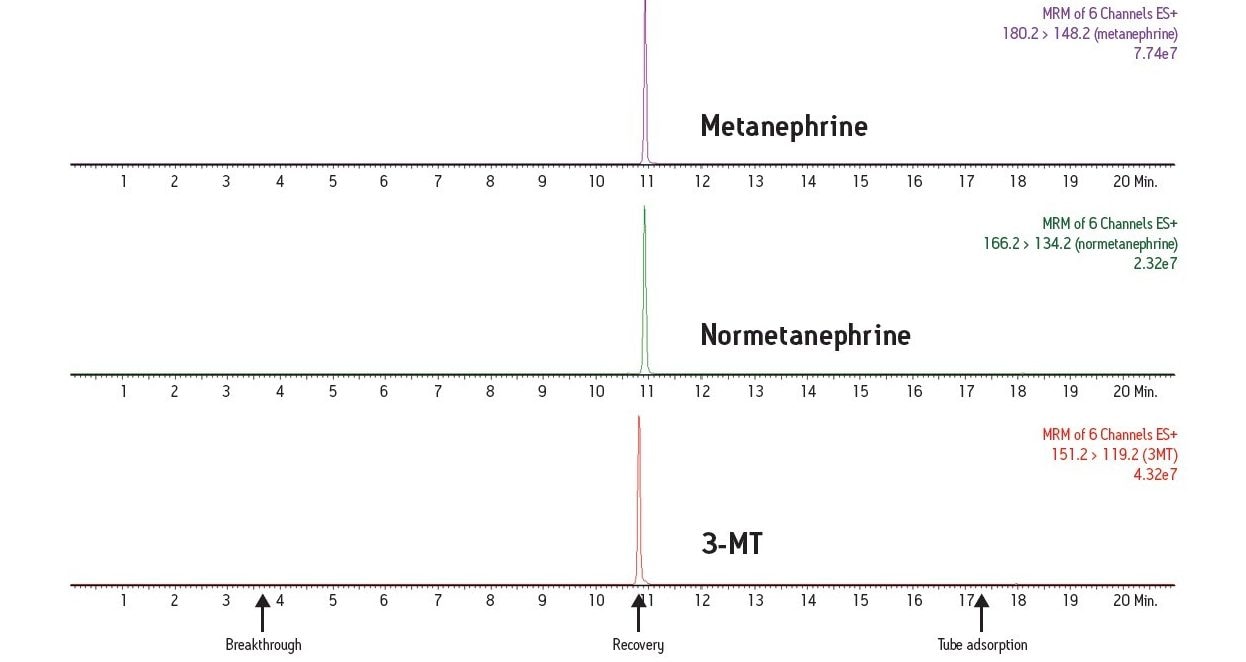

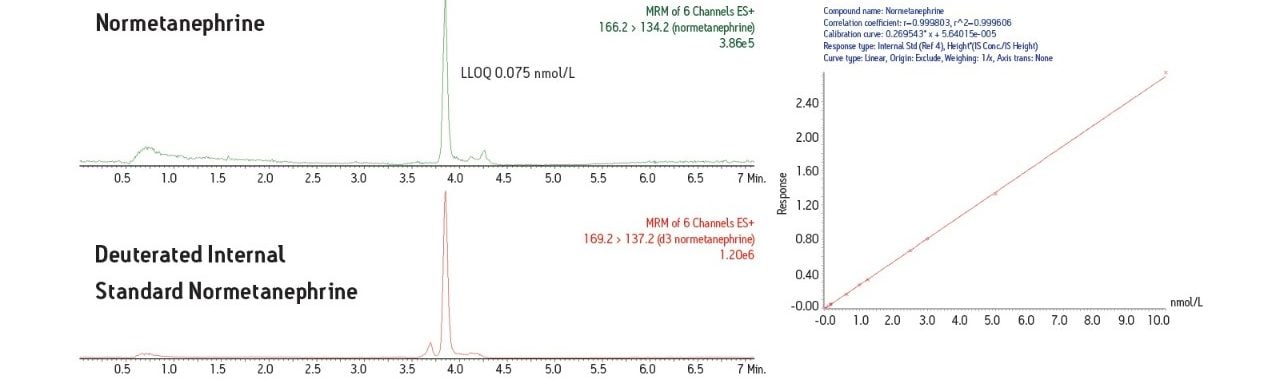
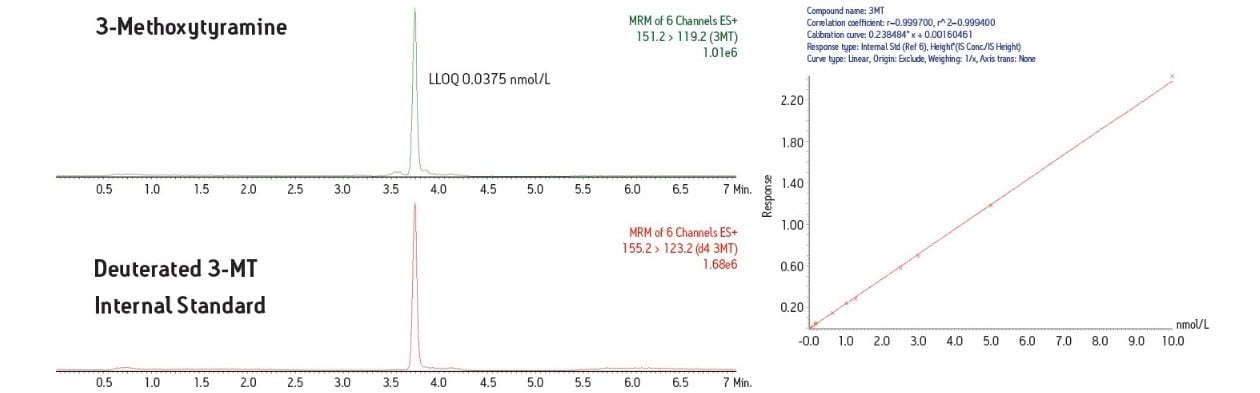
In this study, a clinical research method has been developed for the simultaneous measurement of three plasma biogenic amines. The method utilizes LC-MS and an online SPE system. This combination of SPE sample preparation coupled with the analytical power of LC-MS is able to deliver an efficient assay for these important plasma metanephrines.
The method developed here provides:
720004613, September 2013