For research use only. Not for use in diagnostic procedures.
This application note describes a label-free multi-omics approach that has been applied for the analysis of the transfected human hepatocyte cells by implementing LC-HDMSE (LC-DIA-IM-MS), providing both qualitative and quantitative information within a single experiment.
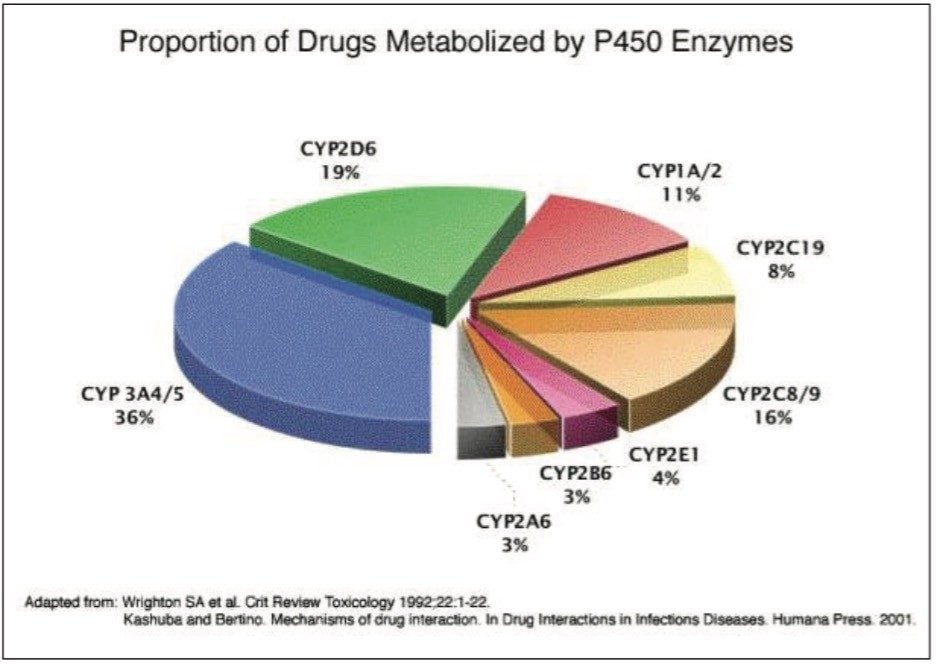
Drug toxicity is a major reason for the failure of candidate pharmaceuticals during their development. Therefore, it is important to realize the potential for toxicity in a timely fashion. Many xenobiotics are bioactivated into toxic metabolites by cytochromes P450 (CYP), as shown in Figure 1. However, the activity of these enzymes typically falls in in vitro systems. Recently, a transformed human hepatocyte cell line (THLE) became available, where the metabolic activity of specific CYP isoforms is maintained. THLE cells could be an ideal system to examine the potential toxicity of candidate pharmaceuticals. The baseline effect of the addition of CYP2E1 gene, which encodes a member of the cytochrome P450 superfamily of enzymes into THLE hepatocytes, has been characterized to better understand the biochemistry of this model system. In this application note, a label-free multi-omics approach has been applied for the analysis of the transfected human hepatocyte cell by implementing LC-HDMSE (LC-DIA-IM-MS), providing both qualitative and quantitative information within a single experiment.
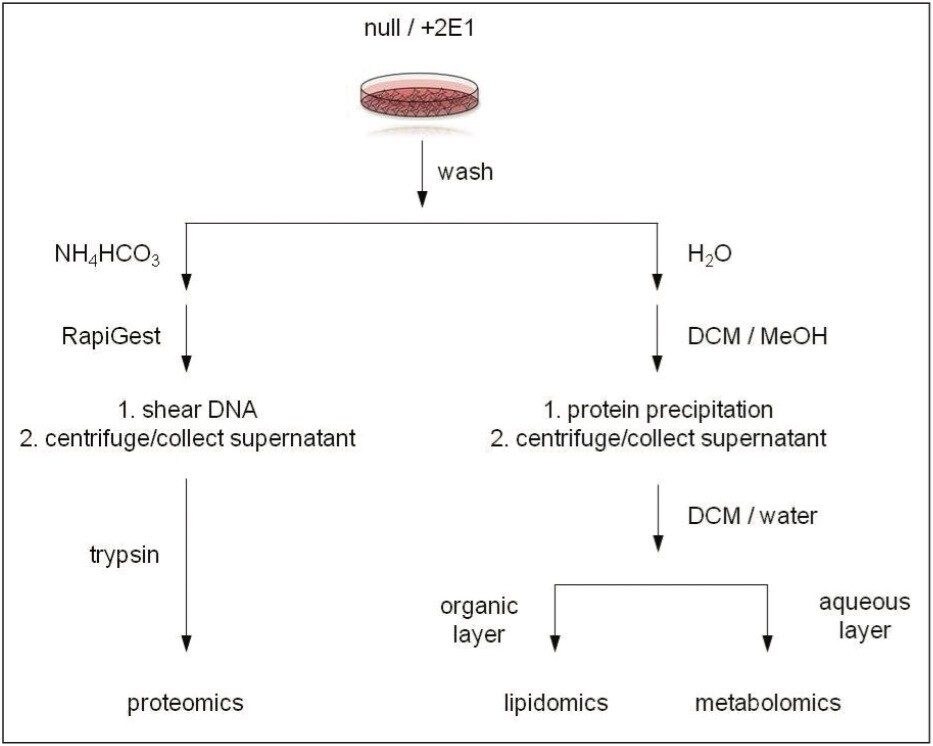
Dedicated and independent sample preparation protocols were applied in order to isolate metabolites, lipids, or proteins, as shown in Figure 2. Three independent replicates of THLE null or THLE+2E1 cells were investigated for all analyte classes. Proteins were recovered and digested with trypsin overnight.
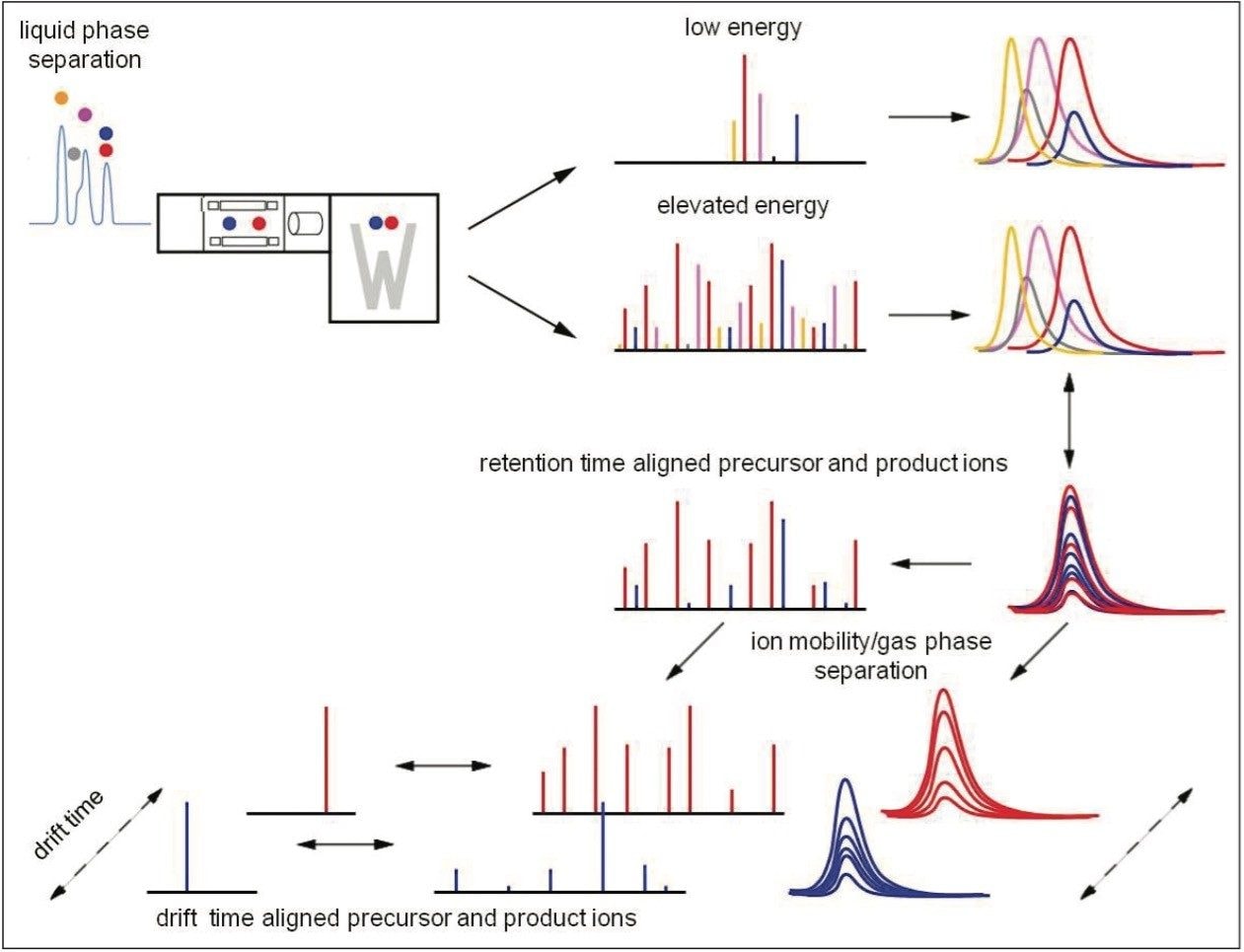
Waters Omics Research Platform Solution with TransOmics Informatics powered by Nonlinear Dynamics was used for all experiments; generic application-dependent LC conditions were applied throughout. In all instances, MS data were acquired using a data independent analysis (DIA) approach, MSE, where the energy applied to the collision cell was switched between a low and elevated energy state during alternate scans. For the proteomics experiments, ion mobility separation (IM) was incorporated into the analytical schema (IM-DIA), HDMSE. The principle of an HDMSE acquisition is shown in Figure 3. Precursor and product ions were associated using dedicated algorithms by retention and/or drift time alignment. For structural elucidation, supplementary MS/MS experiments were conducted for the metabolomics and lipidomics studies.
Label-free LC-MS was used for qualitative and quantitative peptide/protein analyses. Experiments were conducted using a 90 min gradient from 5% to 40% acetonitrile (0.1% formic acid) at 300 nL/min, using a nanoACQUITY UPLC System and an ACQUITY UPLC BEH 1.7 μm C18 reversed phase 75 μm x 20 cm nanoscale LC Column.
For metabolite identification, the LC-MS experiments consisted of a 10 min gradient from 0% to 50% acetonitrile (0.1% formic acid) at 500 μL/min, using an ACQUITY UPLC System. Here, an ACQUITY UPLC BEH 1.7 μm C18 reversed phase 2.1 x 10 cm LC Column was used.
The lipid separations were conducted with a CSH (Charged Surface Hybrid) C18, 1.7 μm, 2.1 x 100 mm Column, also connected to an ACQUITY UPLC System. Mobile phase A consisted of 10 mM NH4HCO2 in ACN/H2O (60/40); and mobile phase B of 10 mM NH4HCO2 in IPA/ACN (90/10). The initial composition of the gradient was 40% B, which was stepped from 43% to 54% B from 2 to 12 min, followed by an additional gradient step from 70% to 99% from 12.1 to 18.0 min. The column flow rate was 400 μL/min and the column temperature maintained at 55 °C.
Data were acquired through data independent analysis (DIA) that utilized a nanoACQUITY UPLC or ACQUITY UPLC System directly interfaced to a hybrid IM-oaToF SYNAPT G2 Mass Spectrometer.
The LC-MS peptide data were processed and searched with ProteinLynx GlobalSERVER v.3.0. Normalized label-free quantification was achieved using TransOmics Informatics Software and additional statistical analysis conducted with Spotfire and EZinfo. The resulting metabolomic and lipidomic data were processed using either MarkerLynx Application Manager or TransOmics Informatics Software, and complementary statistical analysis was conducted with EZinfo.
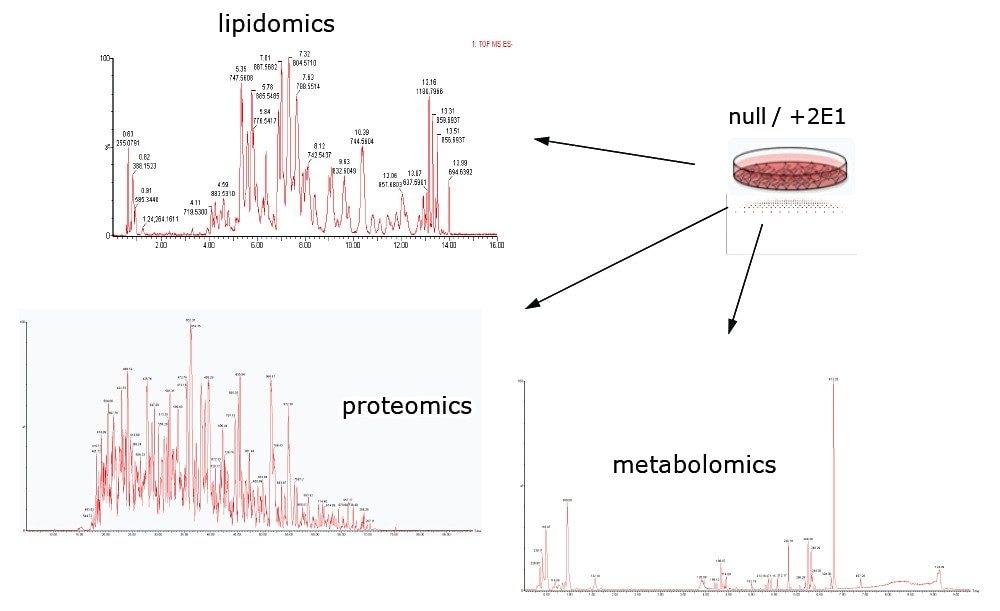
Small amounts of the isolated and purified samples were LC-MS analyzed to identify, quantify, and investigate the metabolomic, lipidomic, and proteomic variances between THLE null (CYP2E1 gene absent) and THLE+2E1 cells. Figure 4 shows the type of chromatographic profiles that were typically obtained for the samples, providing chromatographic definition for downstream analysis of the various data streams. In a similar fashion, spectral profiles were obtained for all sample types and in the instance of the proteomics datasets, ion mobility profiles were obtained as well.
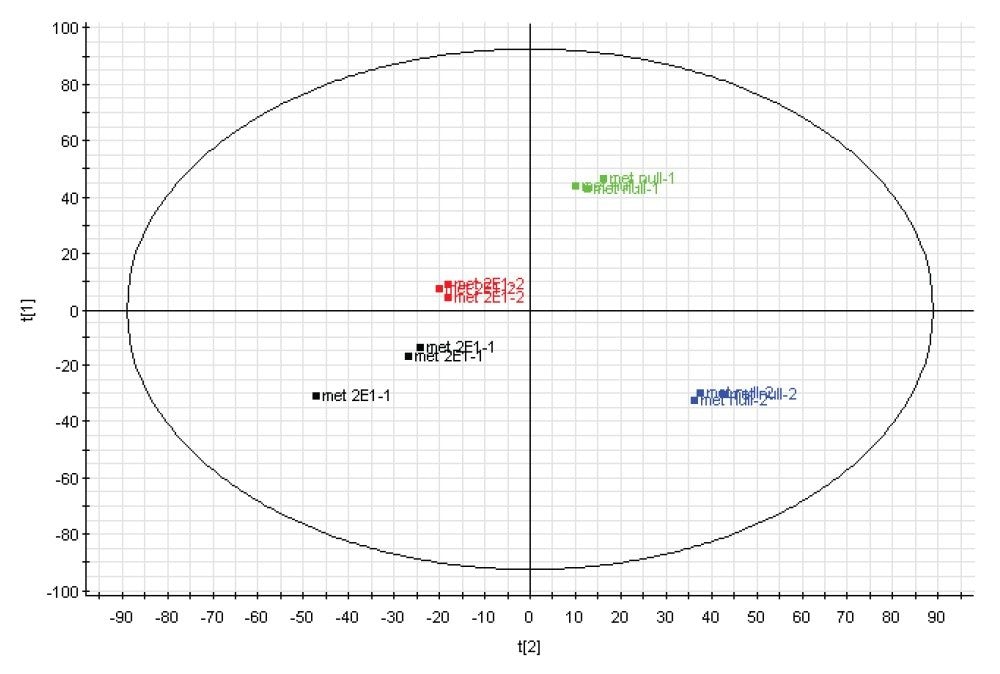
Principal component analysis (PCA) was used in the first instance to identify and highlight significant differences between THLE null and THLE+2E1 cells; an example is shown in Figure 5. For all experiments, good technical LC-MS measurement replication was observed, with slightly greater biological and/or sample preparation variation. The top pane of Figure 5 illustrates group level analysis of the metabolomics data using TransOmics Informatics Software, whereas the bottom pane of Figure 5 demonstrates analysis at the sample level using EZinfo. Further analysis of the data using MarkerLynx Application Manager indicates significant variance in the metabolic expression of guanine and heteropyrithiamine (data not shown). Similar clustering patterns were observed for the lipid, metabolite, and protein datasets.
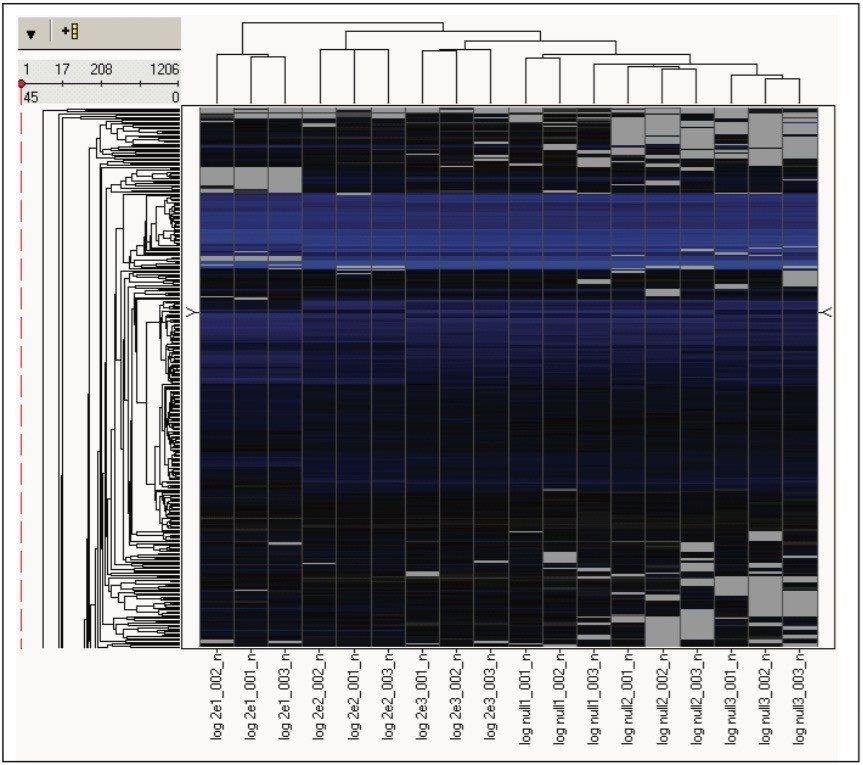
The estimated protein amounts were normalized and exported to facilitate additional statistical analysis at the protein level. First, hierarchical clustering was conducted, which revealed primary grouping at the technical level and secondary grouping at the sample level, as shown in Figure 6. Next, protein regulation values were calculated as a function of sample group level regulation probability. Only the proteins that were identified for which a regulation probability value could be expressed and found to be common to both samples; i.e. THLE null and THLE+2E1 were considered for protein/gene pathway analysis using Ingenuity IPA. The most significantly enriched canonical signalling pathways were EIF2, regulation of EIF4 and p70S6K, mTOR, Actin cytoskeleton, and ILK.
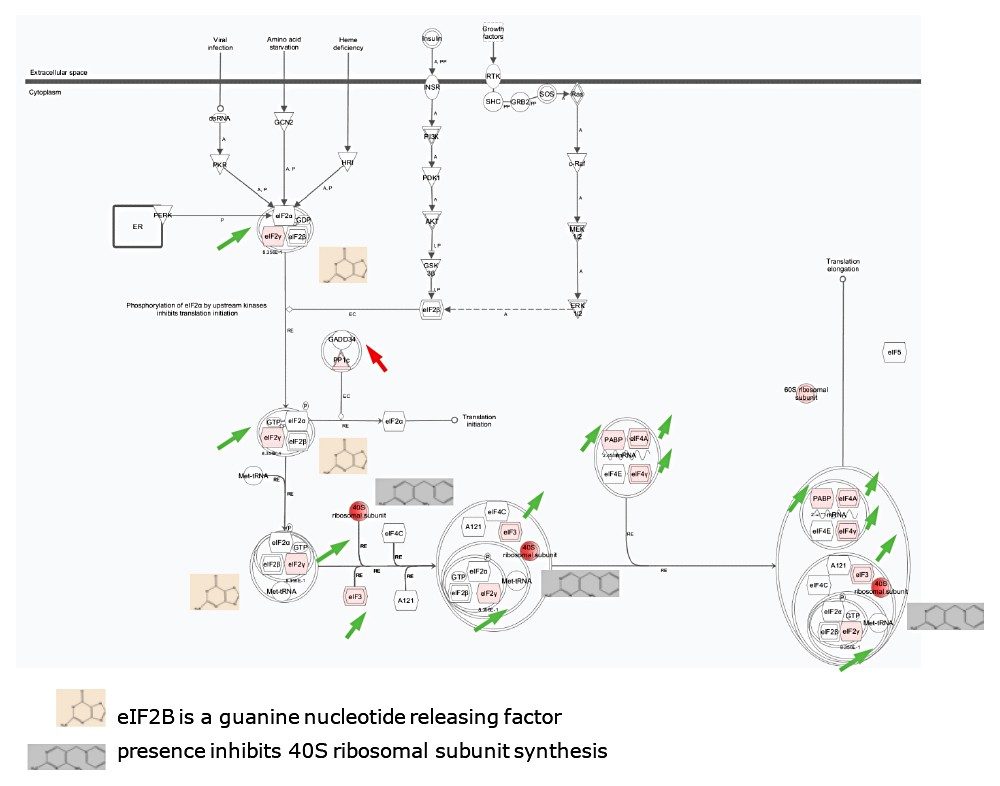
The previously mentioned metabolites interplay with the proteins/genes in the EIF2 (eukaryotic initiation factor 2) signalling pathway, as shown in Figure 7. For example, EIF2B is a guanine nucleotide releasing factor required to release Guanosine diphosphate (GDP), so that a new Guanosine-5'-triphosphate (GTP) molecule can bind and activate EIF2. Moreover, the presence of thiamines is known to inhibit the synthesis of 40S ribosomal subunits. These observations are not unexpected since CYPS readily induce oxidative stress when no substrate is available. Moreover, the EIF2 signalling pathway is one of the primary responders to cellular stress.
A label-free multi-omics approach has been applied for the analysis of the transfected human hepatocyte cells by implementing LC/HDMSE (LC-DIA-IM-MS), providing both qualitative and quantitative information within a single experiment.
720004350, May 2012